Pfizer 2007 Annual Report Download - page 12
Download and view the complete annual report
Please find page 12 of the 2007 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
10 2007 Financial Report
Financial Review
Pfizer Inc and Subsidiary Companies
in cash (including transaction costs). In connection with these
transactions, we recorded $262 million in Acquisition-related
in-process research and development charges.
The following acquisitions, completed in 2008, are not reflected
in our consolidated financial statements as of December 31, 2007:
•In February 2008, we signed an agreement to acquire all issued
and outstanding shares of Encysive Pharmaceuticals Inc. (Encysive),
a biopharmaceutical company with a product (Thelin) for the
treatment of pulmonary arterial hypertension, which is
commercially available in much of the E.U. and is approved in other
markets, as well as other pipeline candidates. Upon completion
of the tender offer, representing an equity value of approximately
$195 million, we will also assume Encysive’s change of control
repurchase obligations under its 2.5% convertible notes.
•In January 2008, we completed the acquisition of all the
outstanding shares of Coley Pharmaceutical Group, Inc. (Coley),
a biopharmaceutical company specializing in vaccines and drug
candidates designed to fight cancers, allergy and asthma disorders,
and autoimmune diseases, for approximately $230 million. In
March 2005, we entered into a license agreement with Coley for
a toll-like receptor 9 (TLR9) agonist for the potential treatment,
control and prevention of cancer. In 2005, we expensed a payment
of $50 million, which was included in Research and development
expenses, and purchased $10 million of Coley’s common stock. In
June 2007, we announced the discontinuation of the development
program associated with this compound.
•In January 2008, we also acquired CovX, a privately-held
biotherapeutics company specializing in preclinical oncology
and metabolic research and the developer of a biotherapeutics
technology platform that we expect will enhance our biologic
portfolio.
Dispositions
We evaluate our businesses and product lines periodically for
strategic fit within our operations. Since January 1, 2005, we
have sold the following businesses:
•In the fourth quarter of 2006, we sold our Consumer Healthcare
business for $16.6 billion, and recorded a gain of approximately
$10.2 billion ($7.9 billion, net of tax) in Gains on sales of
discontinued operations—net of tax in the consolidated
statement of income for 2006. In 2007, we recorded a loss of
approximately $70 million, after-tax, primarily related to the
resolution of contingencies, such as purchase price adjustments
and product warranty obligations, as well as pension
settlements. This business was composed of:
䡬substantially all of our former Consumer Healthcare segment;
䡬other associated amounts, such as purchase-accounting impacts,
acquisition-related costs and restructuring and implementation
costs related to our cost-reduction initiatives that were
previously reported in the Corporate/Other segment; and
䡬certain manufacturing facility assets and liabilities, which
were previously part of our Pharmaceutical or Corporate/
Other segment but were included in the sale of the Consumer
Healthcare business. The net impact to the Pharmaceutical
segment was not significant.
The results of this business are included in Income from
discontinued operations—net of tax for all periods presented.
See Notes to Consolidated Financial Statements—Note 3.
Discontinued Operations.
We continued during 2007, and will continue for a period of time,
to generate cash flows and to report income statement activity
in continuing operations that are associated with our former
Consumer Healthcare business. The activities that give rise to
these impacts are transitional in nature and generally result
from agreements that ensure and facilitate the orderly transfer
of business operations to the new owner. Included in continuing
operations for 2007 were the following amounts associated
with these transition service agreements that will no longer
occur after the full transfer of activities to the new owner:
Revenues of $219 million; Cost of sales of $194 million; Selling,
informational and administrative expenses of $15 million; and
Other (income)/deductions—net of $16 million in income.
•In the third quarter of 2005, we sold the last of three European
generic pharmaceutical businesses, which we had included in
our Pharmaceutical segment, for 4.7 million euro
(approximately $5.6 million). This business became a part of
Pfizer in April 2003 in connection with our acquisition of
Pharmacia. We recorded a loss of $3 million ($2 million, net of
tax) in Gains on sales of discontinued operations—net of tax in
the consolidated statement of income for 2005.
•In the first quarter of 2005, we sold the second of three
European generic pharmaceutical businesses, which we had
included in our Pharmaceutical segment, for 70 million euro
(approximately $93 million). This business became a part of
Pfizer in April 2003 in connection with our acquisition of
Pharmacia. We recorded a gain of $57 million ($36 million, net
of tax) in Gains on sales of discontinued operations—net of tax
in the consolidated statement of income for 2005. In addition,
we recorded an impairment charge of $9 million ($6 million, net
of tax) related to the third European generic business in Income
from discontinued operations—net of tax in the consolidated
statement of income for 2005.
Our Expectations for 2008
While our revenues and income will continue to be tempered in
the near term due to patent expirations and other factors, we will
continue to make the investments necessary to sustain long-term
growth. We remain confident that Pfizer has the organizational
strength and resilience, as well as the financial depth and
flexibility, to succeed in the long term. However, no assurance can
be given that the industry-wide factors described above under
“Our Operating Environment and Response to Key Opportunities
and Challenges” or other significant factors will not have a
material adverse effect on our business and financial results.
Our 2008 guidance reflects the projected impact of the loss of
exclusivity in the U.S. of Norvasc (March 2007) and Zyrtec/Zyrtec
D (January 2008), and the expiration of the U.S. basic patent for
Camptosar (February 2008).
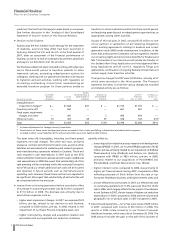
At current exchange rates, we forecast 2008 revenues of $47.0
billion to $49.0 billion, reported diluted earnings per common
share (EPS) of $1.78 to $1.93, Adjusted diluted EPS of $2.35 to
$2.45, and cash flow from operations of $17 billion to $18 billion.