Pfizer 2007 Annual Report Download - page 5
Download and view the complete annual report
Please find page 5 of the 2007 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
approximately $7.9 billion, after tax. (See further discussion in
the “Our Strategic Initiatives—Strategy and Recent Transactions:
Dispositions” and “Analysis of the Consolidated Statement of
Income” sections of this Financial Review.)
•Acquisitions—We completed a number of strategic acquisitions
that we believe will strengthen and broaden our existing
pharmaceutical capabilities. In 2007, we acquired BioRexis, a
privately held biopharmaceutical company with a number of
diabetes candidates and a novel technology platform for
developing new protein drug candidates, and Embrex, an
animal health company that possesses a unique vaccine delivery
system known as Inovoject that improves consistency and
reliability by inoculating chicks while they are still inside the egg.
(See further discussion in the “Our Strategic Initiatives—Strategy
and Recent Transactions: Acquisitions, Licensing and
Collaborations” section of this Financial Review.)
•Cost-reduction initiatives—We made significant progress with
our cost-reduction initiatives, which are a broad-based,
company-wide effort to improve performance and efficiency.
We incurred related costs of approximately $3.9 billion in 2007,
$2.1 billion in 2006 and $763 million in 2005. Building on what
had already been accomplished, in January 2007, we announced
additional plans to change the way we run our business to meet
the challenges of a changing business environment and to
take advantage of the diverse opportunities in the marketplace.
We are generating cost reductions through site rationalizations
in Research and Development (R&D) and manufacturing,
streamlining organizational structures, sales force and staff
function reductions, and increased outsourcing and
procurement savings. (See further discussion in the “Cost-
Reduction Initiatives” section of this Financial Review.)
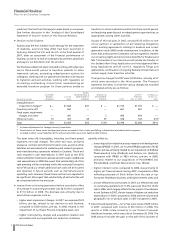
On January 23, 2008, we filed a Current Report on Form 8-K, which
included a press release announcing our fourth-quarter and full-
year 2007 financial results. In completing our final analysis, we
determined that our accruals related to U.S. rebate liabilities
were understated by $195 million, pre-tax, and $154 million,
after-tax. While not material to understanding fourth quarter and
full year 2007 financial results contained in our January 23, 2008,
press release, the amounts disclosed above have been recorded
in our actual results for the fourth quarter and full year 2007. We
believe noting this change is beneficial to understanding our
actual results for the fourth quarter and full year 2007 contained
in this financial report.The impact of this change was as follows:
FOURTH QUARTER 2007 FULL YEAR 2007
_______________________________ ___________________________
PER PER
(MILLIONS, EXCEPT PER JANUARY JANUARY
COMMON SHARE DATA) FORM 8-K ACTUAL FORM 8-K ACTUAL
Revenues $13,065 $12,870 $48,613 $48,418
Net income 2,878 2,724 8,298 8,144
Diluted earnings
per share 0.42 0.40 1.20 1.17
Adjusted income* 3,556 3,402 15,267 15,113
Adjusted diluted
earnings per share* 0.52 0.50 2.20 2.18
* For an understanding of Adjusted income, see the “Adjusted
income” section of this Financial Review.
Our Operating Environment and Response to Key
Opportunities and Challenges
We and our industry continue to face significant challenges in a
profoundly changing business environment, and we are taking
steps to fundamentally change the way we run our businesses to
meet these challenges, as well as to take advantage of the diverse
and attractive opportunities that we see in the marketplace. In
response to these challenges and opportunities, we announced
five priorities in January 2007:
•Maximize our near and long-term revenues;
•Establish a lower and more flexible cost base;
•Create smaller, more focused and more accountable operating
areas;
•Engage more productively with customers, patients, physicians
and other collaborators; and
•Make Pfizer a great place to work.
We believe that we have made progress on all of these goals. For
details about our strategic initiatives, see the “Our Strategic
Initiatives—Strategy and Recent Transactions” section of this
Financial Review, and for details about our cost-reduction initiatives,
see the “Cost-Reduction Initiatives” section of this Financial Review.
There are a number of industry-wide factors that may affect our
business and they should be considered along with the information
presented in the “Forward-Looking Information and Factors That
May Affect Future Results” section of this Financial Review. Such
industry-wide factors include pricing and access, intellectual
property rights, product competition, the regulatory environment,
pipeline productivity and the changing business environment.
Pricing and Access
We believe that our medicines provide significant value for both
healthcare providers and patients, not only from the improved
treatment of diseases, but also from a reduction in other
healthcare costs such as hospitalization or emergency room costs.
Notwithstanding the benefits of our products, the pressures from
governments and other payer groups are continuing and
increasing. These pressure points can include price controls, price
cuts (directly or by rebate actions) and regulatory changes that
limit access to certain medicines.
•Governments around the world continue to seek discounts on
our products, either by leveraging their significant purchasing
power or by mandating prices or implementing various forms
of price controls. The growing power of managed care
organizations in the U.S. has similarly increased the pressure on
pharmaceutical prices and access.
•In the U.S., the enactment of the Medicare Prescription Drug
Improvement and Modernization Act of 2003 (the Medicare
Act), which went into effect in 2006, expanded access to
medicines to patients-in-need through prescription drug benefits
for Medicare beneficiaries. This program has been successfully
implemented, with high levels of beneficiary satisfaction and
lower-than-expected costs to the government due to the
enhanced purchasing power of medical plans in the private
sector to negotiate on behalf of Medicare beneficiaries. Despite
this success, the exclusive role of medical plans in the private
sector in negotiating prices for the Medicare drug benefit
2007 Financial Report 3
Financial Review
Pfizer Inc and Subsidiary Companies