Pfizer 2015 Annual Report Download - page 14
Download and view the complete annual report
Please find page 14 of the 2015 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
2015 Financial Report
13
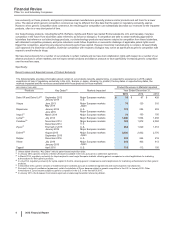
Acquisitions and Fair Value
For a discussion about the application of Fair Value to our recent acquisitions, see “Acquisition of Hospira” below and Notes to Consolidated
Financial Statements—Note 2A. Acquisitions, Licensing Agreements, Collaborative Arrangements, Divestitures, Equity-Method Investments
and Cost-Method Investment: Acquisitions.
For a discussion about the application of Fair Value to our investments, see Notes to Consolidated Financial Statements—Note 7A. Financial
Instruments: Selected Financial Assets and Liabilities.
For a discussion about the application of Fair Value to our benefit plan assets, see Notes to Consolidated Financial Statements––Note 11D.
Pension and Postretirement Benefit Plans and Defined Contribution Plans: Plan Assets.
For a discussion about the application of Fair Value to our asset impairment reviews, see “Asset Impairment Reviews” below.
Revenues
Our gross product revenues are subject to a variety of deductions that are generally estimated and recorded in the same period that the
revenues are recognized, and primarily represent rebates, chargebacks and sales allowances to government agencies, wholesalers/
distributors and managed care organizations with respect to our pharmaceutical products. Those deductions represent estimates of rebates
and discounts related to gross sales for the reporting period, and, as such, knowledge and judgment of market conditions and practice are
required when estimating the impact of these revenue deductions on gross sales for a reporting period.
Historically, our adjustments of estimates, to reflect actual results or updated expectations, have not been material to our overall business. On
a quarterly basis, our adjustments of estimates to reflect actual results generally have been less than 1% of revenues, and have resulted in
either a net increase or a net decrease in revenues. Product-specific rebates, however, can have a significant impact on year-over-year
individual product growth trends. If any of our ratios, factors, assessments, experiences or judgments are not indicative or accurate predictors
of our future experience, our results could be materially affected. The sensitivity of our estimates can vary by program, type of customer and
geographic location. However, estimates associated with U.S. Medicare, Medicaid and performance-based contract rebates are most at risk
for material adjustment because of the extensive time delay between the recording of the accrual and its ultimate settlement, an interval that
can generally range up to one year. Because of this time lag, in any given quarter, our adjustments to actual can incorporate revisions of
several prior quarters.
Asset Impairment Reviews
We review all of our long-lived assets for impairment indicators throughout the year. We perform impairment testing for indefinite-lived
intangible assets and goodwill at least annually and for all other long-lived assets whenever impairment indicators are present. When
necessary, we record charges for impairments of long-lived assets for the amount by which the fair value is less than the carrying value of
these assets. Our impairment review processes are described in the Notes to Consolidated Financial Statements––Note 1K. Basis of
Presentation and Significant Accounting Policies: Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets.
Examples of events or circumstances that may be indicative of impairment include:
• A significant adverse change in legal factors or in the business climate that could affect the value of the asset. For example, a successful
challenge of our patent rights would likely result in generic competition earlier than expected.
• A significant adverse change in the extent or manner in which an asset is used. For example, restrictions imposed by the FDA or other
regulatory authorities could affect our ability to manufacture or sell a product.
• A projection or forecast that indicates losses or reduced profits associated with an asset. This could result, for example, from a change in a
government reimbursement program that results in an inability to sustain projected product revenues and profitability. This also could result
from the introduction of a competitor’s product that results in a significant loss of market share or the inability to achieve the previously
projected revenue growth, as well as the lack of acceptance of a product by patients, physicians and payers. For in-process research and
development (IPR&D) projects, this could result from, among other things, a change in outlook based on clinical trial data, a delay in the
projected launch date or additional expenditures to commercialize the product.
Identifiable Intangible Assets
As a result of our identifiable intangible asset impairment review work, we recognized a number of impairments of identifiable intangible assets
for the years ended December 31, 2015, 2014 and 2013. See Notes to Consolidated Financial Statements––Note 4. Other (Income)/
Deductions—Net.
When we are required to determine the fair value of intangible assets other than goodwill, we use an income approach, specifically the
discounted cash flow method. We start with a forecast of all the expected net cash flows associated with the asset, which includes the
application of a terminal value for indefinite-lived assets, and then we apply an asset-specific discount rate to arrive at a net present value
amount. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net
cash flows, which includes the expected impact of competitive, legal and/or regulatory forces on the projections and the impact of
technological risk associated with IPR&D assets, as well as the selection of a long-term growth rate; the discount rate, which seeks to reflect
the various risks inherent in the projected cash flows; and the tax rate, which seeks to incorporate the geographic diversity of the projected
cash flows.
While all intangible assets other than goodwill can face events and circumstances that can lead to impairment, in general, intangible assets
other than goodwill that are most at risk of impairment include IPR&D assets (approximately $1.2 billion as of December 31, 2015) and newly