Medtronic 2016 Annual Report Download - page 51
Download and view the complete annual report
Please find page 51 of the 2016 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Table of Contents
48
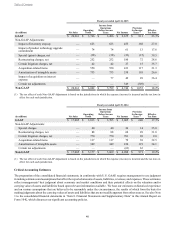
integrates imaging, navigation, and powered surgical instruments. Fiscal year 2015 included several new product launches,
including our Prestige LP cervical disc and Pure Titanium Coated (PTC) interbodies spacers, which partially offset declines in
Core Spine. Interventional Spine net sales decline was driven by a decline in European sales, where the business faced pricing
pressures in Germany and unfavorable currency translation. Underlying demand for BMP stabilized and returned to slight growth
in the latter half of fiscal year 2015.
Neuromodulation net sales for fiscal year 2015 were $2.0 billion, an increase of 4 percent over the prior fiscal year. The increase
in net sales was primarily due to strong growth in Gastro/Uro and growth in DBS and Pain Stimulation. Our global focus on our
neurologist referral programs, and the strength of the EARLYSTIM data in international markets, continues to drive solid growth
of DBS systems. Implant growth of our InterStim Therapy for overactive bladder, urinary retention, and bowel incontinence
continued in the U.S. throughout fiscal year 2015. The increase in net sales for fiscal year 2015 was also due to global growth of
our RestoreSensor SureScan MRI system. While the U.S. pain stimulation market has weakened as a result of reimbursement
changes, net sales of our SureScan MRI system for the fiscal year demonstrate our continued strength in the market.
Surgical Technologies net sales for fiscal year 2015 were $1.7 billion, an increase of 7 percent over the prior fiscal year. The
increase in net sales was driven by continued worldwide net sales growth across the portfolio of Advanced Energy, ENT, and
Neurosurgery, partially offset by unfavorable currency translation. Performance was driven by strong growth of power systems,
Aquamantys Transcollation, and PEAK PlasmaBlade technologies, as well as solid growth of Midas Rex products, monitoring,
and O-arm imaging systems. Additionally, net sales growth was positively impacted by launch of our NuVent sinus balloons in
the second quarter of fiscal year 2015 and the acquisition of Visualase during the first quarter of fiscal year 2015, adding a MRI-
guided laser ablation technology to our broad suite of neuroscience solutions for neurosurgery. The increase in revenue from
Visualase and our NuVent sinus balloons was partially offset by our divestiture of the MicroFrance product line during the third
quarter of fiscal year 2015.
Neurovascular net sales for fiscal year 2015 were $132 million. The division, formerly part of Covidien, contributed revenue from
the strength of its coils, stents, flow diversion, and access product lines. The New England Journal of Medicine published several
positive clinical trials on our Solitaire FR revascularization device, resulting in continued customer adoption of the product.
Additionally, net sales were positively impacted by the U.S. launch of the Pipeline Flex embolization device, which was launched
during the third quarter of fiscal year 2015.
Looking ahead, we expect our Restorative Therapies Group could be affected by the following:
• Changes in procedural volumes, competitive and pricing pressure, reimbursement challenges, impacts from
changes in the mix of our product offerings, the timing of product registration approvals, and fluctuations in
currency exchange rates.
• Continued commercial integration in the Restorative Therapies group and market acceptance of our new
integrated solutions through the Surgical Synergy program, which integrates our spinal implants and Surgical
Technologies' imaging and navigation equipment.
• Market acceptance and continued adoption of innovative new products, such as our CD Horizon Solera Voyager
system, our ELEVATE expandable interbody cage, and our OLIF25 and OLIF51 procedure solutions, both of
which have recently been augmented with new implant technology.
• Continued pricing and competitive pressures on premium balloon kyphoplasty (BKP) within Interventional
Spine. Though we remain focused on communicating the clinical and economic benefits for premium BKP,
we expect pressure in several markets to continue. We believe opportunities for growth exist in the broader
vertebral compression fracture (VCF) and adjacent markets, and continue to pursue the development of other
therapies to treat more patients with VCF, including the recent U.S. launches of both the Kyphon V vertebroplasty
system and the Osteocool tumor ablation system.
• Acceptance of Kanghui's broad portfolio of trauma, spine, and large-joint reconstruction products focused on
the growing global value segment.
• Continued acceptance and adoption rates of stimulators and leads approved to treat chronic pain in major
markets around the world.
• Ongoing obligations under the U.S. FDA consent decree entered in April 2015 relating to the SynchroMed
drug infusion system and the Neuromodulation quality system. We continue to make progress against our U.S
FDA consent decree commitments.
• Continued and future acceptance of our current indications for Medtronic DBS Therapy for the treatment of
movement disorders, epilepsy (approved in Europe), and OCD. The DBS Therapy portfolio includes Activa