Medtronic 2016 Annual Report Download - page 45
Download and view the complete annual report
Please find page 45 of the 2016 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Table of Contents
42
The Company assesses the impairment of indefinite-lived intangibles annually in the third quarter and whenever an event occurs
or circumstances change that would indicate that the carrying amount may be impaired. Our impairment tests of indefinite-lived
intangibles require the Company to make several estimates about fair value, most of which are based on projected future cash
flows. Indefinite-lived intangible assets, were $721 million and $720 million as of April 29, 2016 and April 24, 2015, respectively.
The results of our annual impairment test are discussed in Note 6 to the consolidated financial statements in “Item 8. Financial
Statements and Supplementary Data” in this Annual Report on Form 10-K.
Contingent Consideration Contingent consideration is recorded at the acquisition date at estimated fair value and is remeasured
each reporting period with the change in fair value recognized as income or expense within acquisition-related items in our
consolidated statements of income. Changes to the fair value of contingent consideration can result from changes in the timing
and amount of revenue estimates, in the timing or probability of achieving the milestones which trigger payment, or in discount
rates. The fair value of contingent consideration was $377 million and $264 million as of April 29, 2016 and April 24, 2015,
respectively.
Net Sales
In the fourth quarter of fiscal year 2015, we amended the way in which we evaluate performance and allocate resources with the
acquisition of Covidien. As a result, we began to operate under four reportable segments and four operating segments, the Cardiac
and Vascular Group (composed of Cardiac Rhythm & Heart Failure, Coronary & Structural Heart and Aortic & Peripheral Vascular
businesses), the Minimally Invasive Therapies Group (composed of Surgical Solutions and Patient Monitoring & Recovery), the
Restorative Therapies Group (composed of the Spine, Neuromodulation, Surgical Technologies, and Neurovascular businesses),
and the Diabetes Group. See Note 17 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary
Data” in this Annual Report on Form 10-K for additional discussion related to our segment reporting.
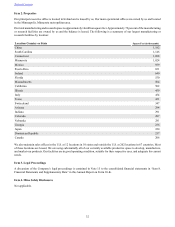
The table below illustrates net sales by operating segment and division for fiscal years 2016, 2015, and 2014:
Net Sales Net Sales
Fiscal Year Fiscal Year
(dollars in millions; NC - Not Calculable) 2016 2015 % Change 2015 2014 % Change
Cardiac Rhythm & Heart Failure $ 5,465 $ 5,245 4 % $ 5,245 $ 4,996 5 %
Coronary & Structural Heart 3,093 3,038 2 3,038 2,956 3
Aortic & Peripheral Vascular (1) 1,638 1,078 52 1,078 895 20
Total Cardiac and Vascular Group 10,196 9,361 9 9,361 8,847 6
Surgical Solutions (1) 5,265 1,293 307 1,293 — NC
Patient Monitoring & Recovery (1) 4,298 1,094 293 1,094 — NC
Total Minimally Invasive Therapies Group (1) 9,563 2,387 301 2,387 — NC
Spine 2,924 2,971 (2) 2,971 3,041 (2)
Neuromodulation 1,926 1,977 (3) 1,977 1,898 4
Surgical Technologies 1,773 1,671 6 1,671 1,562 7
Neurovascular (1) 587 132 345 132 — NC
Total Restorative Therapies Group 7,210 6,751 7 6,751 6,501 4
Diabetes Group 1,864 1,762 6 1,762 1,657 6
Total $ 28,833 $ 20,261 42% $ 20,261 $ 17,005 19%
(1) Growth rates are impacted by the acquisition of Covidien in the fourth quarter of fiscal year 2015. Revenue growth is compared to a full
year of operations in fiscal year 2016.
Cardiac and Vascular Group The Cardiac and Vascular Group’s products, with specific focus on comprehensive disease
management, include pacemakers, insertable and external cardiac monitors, cardiac resynchronization therapy devices (CRT-D),
implantable cardioverter defibrillators (ICD), leads and delivery systems, ablation products, electrophysiology catheters, products
for the treatment of atrial fibrillation, information systems for the management of patients with Cardiac Rhythm & Heart Failure
devices, products designed to reduce surgical site infections, coronary and peripheral stents, balloon, and related delivery systems,
endovascular stent graft systems, heart valve replacement technologies, cardiac tissue ablation systems, and open heart and coronary
bypass grafting surgical products. The Cardiac and Vascular Group also includes Care Management Services (formerly known as
Cardiocom) and Cath Lab Managed Services (CLMS) within the Cardiac Rhythm & Heart Failure division. The Cardiac and