Medtronic 2008 Annual Report Download - page 26
Download and view the complete annual report
Please find page 26 of the 2008 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
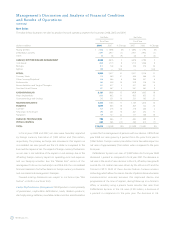
Net Sales
The table below illustrates net sales by product line and operating segment for fiscal years 2008, 2007, and 2006:
Net Sales Net Sales
Fiscal Year Fiscal Year
(dollars in millions) 2008 2007 % Change 2007 2006 % Change
Pacing Systems $ 2,008 $ 1,895 6% $ 1,895 $ 1,795 6%
Defibrillation Systems 2,897 2,917 (1) 2,917 2,932 (1)
Other 58 64 (9) 64 67 (4)
CARDIAC RHYTHM DISEASE MANAGEMENT 4,963 4,876 2 4,876 4,794 2
Core Spinal 1,869 1,713 9 1,713 1,566 9
Biologics 815 704 16 704 570 24
Kyphon 298 — N/A — — N/A
SPINAL 2,982 2,417 23 2,417 2,136 13
Coronary Stents 710 560 27 560 366 53
Other Coronary/Peripheral 408 386 6 386 357 8
Endovascular 285 259 10 259 216 20
Revascularization and Surgical Therapies 431 417 3 417 401 4
Structural Heart Disease 297 287 3 287 263 9
CARDIOVASCULAR 2,131 1,909 12 1,909 1,603 19
Neuro Implantables 1,069 962 11 962 833 15
Gastroenterology and Urology 242 221 10 221 183 21
NEUROMODULATION 1,311 1,183 11 1,183 1,016 16
DIABETES 1,019 863 18 863 722 20
Core ENT 323 278 16 278 266 5
Neurologic Technologies 298 261 14 261 235 11
Navigation 159 127 25 127 108 18
SURGICAL TECHNOLOGIES 780 666 17 666 609 9
PHYSIO-CONTROL 329 385 (15) 385 412 (7)
TOTAL
$ 13,515
$ 12,299
10
%
$ 12,299
$ 11,292
9
%
In fiscal years 2008 and 2007, net sales were favorably impacted
by foreign currency translation of $400 million and $166 million,
respectively. The primary exchange rate movements that impact our
consolidated net sales growth are the U.S. dollar as compared to the
Euro and the Japanese Yen. The impact of foreign currency fluctuations
on net sales is not indicative of the impact on net earnings due to the
offsetting foreign currency impact on operating costs and expenses
and our hedging activities. See the “Market Risk” section of this
management’s discussion and analysis and Note 8 to the consolidated
financial statements for further details on foreign currency instruments
and our related risk management strategies.
Forward-looking statements are subject to risk factors (see “Risk
Factors” set forth in our Form 10-K).
Cardiac Rhythm Disease Management CRDM products consist primarily
of pacemakers, implantable defibrillators, leads, ablation products,
electrophysiology catheters, insertable cardiac monitors and information
systems for the management of patients with our devices. CRDM fiscal
year 2008 net sales grew by 2 percent from the prior fiscal year to
$4.963 billion. Foreign currency translation had a favorable impact on
net sales of approximately $160 million when compared to the prior
fiscal year.
Defibrillation Systems net sales of $2.897 billion for fiscal year 2008
decreased 1 percent as compared to fiscal year 2007. The decrease in
net sales is the result of sales declines in the U.S., offset by sales growth
outside the U.S. Global sales were driven by the Virtuoso ICD and the
Concerto CRT-D. Both of these devices feature Conexus wireless
technology which allows for remote transfer of patient data and enables
communication remotely between the implanted device and
programmer at the time of implant, during follow-up in a clinician’s
office, or remotely using a patient home monitor. Net sales from
Defibrillation Systems in the U.S. were $1.955 billion, a decrease of
6 percent in comparison to the prior year. The decrease in U.S.
Management’s Discussion and Analysis of Financial Condition
and Results of Operations
(continued)
22 Medtronic, Inc.