Medtronic 2008 Annual Report Download - page 33
Download and view the complete annual report
Please find page 33 of the 2008 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
represents the first time patients with diabetes have been able to
use a low-glucose suspend feature, the development of which is
considered by many in the industry to be a major advance towards
a closed-loop diabetes management system.
Surgical Technologies Surgical Technologies products are used to treat
conditions of the ear, nose and throat, and certain neurological
disorders. Additionally, we manufacture and sell image-guided surgery
systems. Our portfolio consists of powered tissue-removal systems and
other microendoscopy instruments, implantable devices, nerve
monitoring systems, disposable fluid-control products, a Ménière’s
disease therapy device, hydrocephalus shunt devices, external drainage
systems, cranial fixation devices, neuroendoscopes, dura repair products
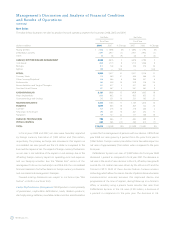
and image-guided surgery systems. Surgical Technologies net sales for
fiscal year 2008 increased by 17 percent over the prior fiscal year to
$780 million. Foreign currency translation had a favorable impact of
$20 million on net sales when compared to the prior fiscal year.
Core ENT net sales grew 16 percent to $323 million in fiscal year 2008
led by strong growth of sales outside the U.S. of the Straightshot M4
Microdebrider and endoscopy sales. In the U.S., there was an increase
in net sales of our Image Guided Surgery Systems which was partially
due to the launch of the Fusion EM IGS System for use in sinus surgical
procedures. Fusion EM IGS is an electromagnetic-based image-guided
surgery product that will avoid “line of sight constraints” of optical
systems. Net sales of monitoring disposables also experienced strong
worldwide growth.
Neurologic Technologies net sales grew 14 percent to $298 million
in fiscal year 2008. The primary drivers of growth in Neurologic
Technologies were continued acceptance of high-speed powered
surgical drill systems, including the EHS Stylus system.
Navigation net sales for fiscal year 2008 increased 25 percent from
the prior fiscal year to $159 million based on strong U.S. net sales of the
O-arm Imaging Systems, a multi-dimensional surgical imaging platform
that is optimized for use in spine and orthopedic surgery, and increased
global service revenue.
Surgical Technologies net sales for fiscal year 2007 increased by
9 percent over the prior fiscal year to $666 million. Foreign currency
translation had a favorable impact of $8 million on net sales when
compared to the prior fiscal year.
Core ENT net sales grew 5 percent to $278 million in fiscal year 2007
led by continued physician acceptance of the Straightshot M4
Microdebrider and the NIM-Response 2.0 Nerve Integrity Monitor.
Net sales within Core ENT were impacted by the loss of revenue from
our tonometry product line, which was sold in the third quarter of fiscal
year 2006.
Neurologic Technologies net sales grew 11 percent to $261 million in
fiscal year 2007. The primary drivers of growth in Neurologic Technologies
were continued acceptance of high-speed powered surgical drill
systems, including the EHS Stylus system and the Strata valve used in
the treatment of hydrocephalus. The Strata valve is an adjustable
flow control valve in which the resistance properties of the valve
can be charged non-invasively by the caregiver. The valve is designed
to minimize overdrainage of cerebrospinal fluid and maintain
intraventricular pressure within a normal physiologic range, regardless
of patient position.
Navigation net sales for fiscal year 2007 increased 18 percent from
fiscal year 2006 to $127 million based on strong sales of the PoleStar
N20, an intra-operative Magnetic Resonance Image (iMRI)-Guidance
System and O-arm Imaging Systems.
Looking ahead, we expect our Surgical Technologies operating
segment should benefit from the following:
• Continued acceptance of our new FUSION EM IGS System that was
launched in the U.S. in the third quarter of fiscal year 2008.
• Continued adoption of power systems outside the U.S. for sinus
procedures, including the Straightshot M4 Microdebrider, as well
as continued global adoption of nerve monitoring for ENT and
thyroid procedures.
• Continued development of the normal pressure hydrocephalus
market, resulting in increased sales of our shunt products, including
the Strata valve, and continued acceptance of our Legend high-
speed drill systems, electric bone mill, and Durepair dura
substitute.
• Continued acceptance of the O-arm Imaging System and future
acceptance of the S7 Navigation System which we expect to release
in fiscal year 2009.
Continued net sales growth in all operating segments is contingent
on our ability to gain further market share, penetrate existing
markets, develop new products, improve existing products and develop
new markets.
29Medtronic, Inc.