Medtronic 2011 Annual Report Download - page 10
Download and view the complete annual report
Please find page 10 of the 2011 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
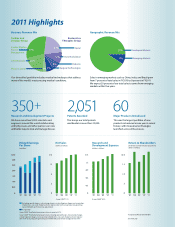
6
Renal denervation* delivers
low-power radio frequency
energy to sympathetic nerves
just outside the kidneys,
essentially disabling them from
sending the signals that cause
high blood pressure.
People with high blood pressure are at increased risk of developing life-
threatening conditions such as stroke or heart attack. Yet for hundreds of
millions of sufferers, first-line therapies — diet, exercise, and medication —
aren’t enough to bring their blood pressure under control. So we invested in
an innovative, catheter-based treatment* for high blood pressure.
I am the face of prevention.
The treatment, called renal denervation, addresses
a key contributor to high blood pressure, also known
as hypertension: overactive sympathetic nerves.
Renal denervation uses low-power radio frequency
energy to selectively silence these nerves leading
into and out of the kidneys.
This pioneering procedure is minimally invasive and
doesn’t require an implant. Studies† have shown that
renal denervation, in combination with medication,
resulted in a signicant and sustained drop in blood
pressure compared with medication alone.
To make renal denervation available to more patients
with uncontrolled hypertension, we recently purchased
Ardian, a California-based company that already had
the technology for this treatment on the market in some
geographies. Its Symplicity catheter system became
available commercially in Europe and Australia in 2010
and has been enthusiastically welcomed by the medical
community. We plan to begin a U.S. clinical trial of
renal denervation for the treatment of uncontrolled
hypertension in late 2011.
While treating uncontrolled hypertension alone makes
this therapy a worthwhile investment, renal denervation
also holds promise for other conditions that stem from
overactive sympathetic nerves, such as kidney failure,
diabetes, and heart failure.
* Not approved for commercial distribution in the United States.
† See page 99 for references.