Medtronic 2011 Annual Report Download - page 67
Download and view the complete annual report
Please find page 67 of the 2011 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
63
Medtronic, Inc.
In connection with acquisitions of ATS Medical and Osteotech,
the Company began to assess and formulate a plan for the
elimination of duplicative positions and the termination of certain
contractual obligations. As a result, the Company incurred
approximately $24 million and $21 million, respectively, of certain
acquisition-related costs, which include legal fees and severance
costs, change in control costs, and contract termination costs,
which were recorded within acquisition-related items in the
consolidated statement of earnings.
Fiscal Year 2010
Invatec S.p.A. In April 2010, the Company acquired privately-held
Invatec S.p.A. (Invatec), a developer of innovative medical
technologies for the interventional treatment of cardiovascular
disease, and two affiliated companies. Invatec’s two affiliated
companies are Fogazzi, which provides polymer technology to
Invatec; and Krauth Cardiovascular, which distributes Invatec
products in Germany. Under the terms of the agreement, the
transaction included an initial up-front payment of $350 million,
which includes the assumption and settlement of existing Invatec
debt. The agreement also includes potential additional payments
of up to $150 million contingent upon achievement of certain
milestones. Total consideration for the transaction was valued
at approximately $468 million, which includes the $350 million
up-front payment plus the estimated fair value of additional
milestone-based contingent consideration of $118 million.
The potential contingent payments consist of up to $75 million
upon reaching a revenue milestone in fiscal year 2011 and up to
$75 million upon reaching a product development milestone by
fiscal year 2013. The Company has recorded, as of the acquisition
date, the estimated fair value of the contingent milestone
payments of $118 million as a component of the consideration
transferred as part of the acquisition of Invatec.
In connection with the acquisition of Invatec, the Company
acquired $228 million of technology-based intangible assets with
an estimated useful life of 12 years. Also as part of the acquisition,
the Company recorded $114 million and $161 million of IPR&D
and goodwill, respectively. The value attributable to IPR&D has
been capitalized as an indefinite-lived intangible asset. The IPR&D
primarily relates to the future launch of Invatec’s drug-eluting
balloons into the U.S. market. Development costs incurred on
the project, estimated to be approximately $44 million, will be
expensed as incurred. The establishment of goodwill was primarily
due to the expected revenue growth that is attributable to
increased market penetration from future products and customers.
The goodwill is not deductible for tax purposes.
The Company has accounted for the acquisition of Invatec as
a business combination. The purchase price has been allocated
as follows:
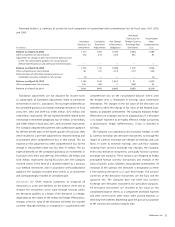
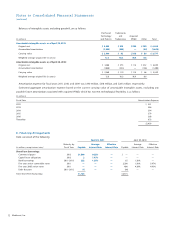
(in millions)
Current assets $ 77
Property, plant, and equipment 32
IPR&D 114
Other intangible assets 228
Goodwill 161
Other assets 1
Total assets acquired 613
Current liabilities 46
Long-term deferred tax liabilities, net 99
Total liabilities assumed 145
Net assets acquired $ 468
Other Acquisitions and Acquisition-Related Items In connection with
the acquisition of Invatec, the Company began to assess and
formulate a plan for the elimination of duplicative positions and
the termination of certain contractual obligations. As a result, the
Company incurred approximately $12 million of acquisition-
related costs in fiscal year 2010. In February 2010, the Company
recorded an IPR&D charge of $11 million related to the asset
acquisition of Arbor Surgical Technologies, Inc.’s bovine pericardial
heart valve technology. These amounts were recorded within
acquisition-related items in the consolidated statement of earnings.
In August 2009, the Company acquired certain intangible assets
related to the distribution of coronary products within the
CardioVascular Japan business. In connection with the acquisition,
the Company recorded $29 million of intangible assets with an
estimated useful life of five years.
Fiscal Year 2009
CoreValve, Inc. In April 2009, the Company acquired CoreValve Inc.
(CoreValve), a privately-held company. Under the terms of the
agreement announced in February 2009, the transaction included
an initial up-front payment, including direct acquisition costs,
of $700 million plus potential additional payments contingent
upon achievement of certain clinical and revenue milestones.
CoreValve develops percutaneous, catheter-based transfemoral
aortic valve replacement products that are approved in certain
markets outside the U.S.