Medtronic 2011 Annual Report Download - page 100
Download and view the complete annual report
Please find page 100 of the 2011 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
96 Medtronic, Inc.
Notes to Consolidated Financial Statements
(continued)
2008 civil investigative demand from the Massachusetts Attorney
General’s Office and several inquiries from the United States
Senate. The Company is fully cooperating with these investigations.
In late June 2008, the Company received a subpoena issu ed
by the United States Attorney’s Office for the District of
Massachusetts pursuant to HIPAA, relating to the Company’s
marketing of biliary stents. The Company is fully cooperating with
this inquiry. On February 19, 2010, a complaint captioned United
States of America ex rel Tricia Nowak and Enda Dodd v. Medtronic,
filed in the United States District Court for the District of
Massachusetts and relating to similar issues was unsealed. On
April 23, 2010, Medtronic filed a motion to dismiss the complaint.
On September 25, 2007 and November 16, 2007, the Company
received letters from the U.S. Securities and Exchange Commission
(SEC) and U.S. Department of Justice, respectively, requesting
information relating to any potential violations of the U.S. Foreign
Corrupt Practices Act in connection with the sale of medical
devices in several non-U.S. countries. A number of competitors
have publicly disclosed receiving similar letters. Subsequently, the
SEC and Department of Justice have made additional requests for
information from the Company. The Company is fully cooperating
with the requests.
On October 24, 2005, the Company received a subpoena from
the United States Attorney’s Office for the District of Massachusetts
issued under HIPAA requesting documents the Company may
have, if any, relating to pacemakers and defibrillators and related
components; monitoring equipment and services; a provision of
benefits, if any, to persons in a position to recommend purchases
of such devices; and the Company’s training and compliance
materials relating to the fraud and abuse and federal Anti-
Kickback statutes. In September 2008, the United States Attorney’s
office for the District of Massachusetts informed Medtronic that
it is no longer pursuing its investigation of Medtronic related to
the October 24, 2005 subpoena. On September 5, 2008, Medtronic
received a subpoena from the Office of Inspector General for
the Department of Health and Human Services in the District
of Minnesota, requesting production of substantially the same
materials covered in the 2005 Massachusetts subpoena. The
Company is fully cooperating with this inquiry.
In accordance with U.S. GAAP, during fiscal year 2011, the
Company recorded $24 million in expense related to probable
and reasonably estimated damages in connection with these
subpoenas.
In the normal course of business, the Company periodically
enters into agreements that require it to indemnify customers or
suppliers for specific risks, such as claims for injury or property
damage arising out of the Company’s products or the negligence
of its personnel or claims alleging that its products infringe third-
party patents or other intellectual property. The Company’s
maximum exposure under these indemnification provisions
cannot be estimated, and the Company has not accrued any
liabilities within the consolidated financial statements. Historically,
the Company has not experienced significant losses on these
types of indemnifications.
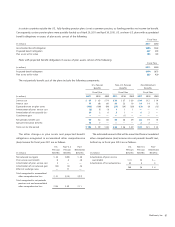
17. Quarterly Financial Data (unaudited)
(in millions, except
per share data)
First
Quarter
Second
Quarter
Third
Quarter
Fourth
Quarter
Fiscal
Year
Net Sales
2011 $3,773 $3,903 $3,961 $4,295 $ 15,933
2010 3,933 3,838 3,851 4,196 15,817
Gross Profit
2011 $2,880 $2,942 $2,975 $3,225 $ 12,021
2010 2,967 2,916 2,939 3,184 12,005
Net Earnings
2 011 $ 830 $ 566 $ 924 $ 776 $ 3,096
2010 445 868 831 954 3,099
Basic Earnings
per Share
2011 $ 0.76 $ 0.52 $ 0.86 $ 0.73 $ 2.87
2010 0.40 0.78 0.75 0.87 2.80
Diluted Earnings
per Share
2011 $ 0.76 $ 0.52 $ 0.86 $ 0.72 $ 2.86
2010 0.40 0.78 0.75 0.86 2.79
The data in the schedule above has been intentionally rounded
to the nearest million and therefore the quarterly amounts may
not sum to the fiscal year-to-date amounts.
18. Segment and Geographic Information
In December 2009, the Company consolidated its businesses
into two operating groups. This structure further advances the
Company’s goal to capitalize on existing synergies related to
customers and technologies across each business. The creation of
these two operating groups did not immediately change how the
Company internally managed and reported the results of these
businesses in fiscal year 2010. Starting in the first quarter of fiscal
year 2011, due to changes in how the Company internally manages
and reports the results of these businesses, the Company began
to operate under two reportable segments and two operating
2011