LabCorp 2007 Annual Report Download - page 30
Download and view the complete annual report
Please find page 30 of the 2007 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
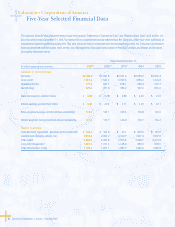
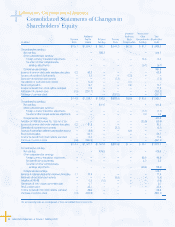
Management’s Discussion and Analysis of Financial
Condition and Results of Operations (Dollars in millions)
28 Laboratory Corporation of America® Holdings 2007
2) adverse results from investigations of clinical laboratories by the
government, which may include signifi cant monetary damages
and/or exclusion from the Medicare and Medicaid programs;
3) loss or suspension of a license or imposition of a fi ne or penalties
under, or future changes in, or interpretations of, the law or
regulations of the Clinical Laboratory Improvement Act of 1967,
and the Clinical Laboratory Improvement Amendments of 1988,
or those of Medicare, Medicaid, the False Claims Act or other
federal, state or local agencies;
4) failure to comply with the Federal Occupational Safety and
Health Administration requirements and the Needlestick
Safety and Prevention Act, which may result in penalties and
loss of licensure;
5) failure to comply with HIPAA, including the failure to meet new
NPI requirements, which could result in denial of claims and/or
signifi cant fi nes;
6) failure of third-party payers to complete testing with the Company,
or accept or remit transactions in HIPAA-required standard
transaction and code set format (including a National Provider
Identifi er), could result in an interruption in the Company’s
cash fl ow;
7) increased competition, including competition from companies
that do not comply with existing laws or regulations or otherwise
disregard compliance standards in the industry;
8) increased price competition, competitive bidding for laboratory
tests and/or changes or reductions to fee schedules;
9) changes in payer mix, including an increase in capitated managed-
cost health care or the impact of a shift to consumer-driven
health plans;
10) failure to obtain and retain new customers and alliance
partners, or a reduction in tests ordered or specimens
submitted by existing customers;
11) failure to retain or attract managed care business as a result of
changes in business models, including new risk based or network
approaches, or other changes in strategy or business models by
managed care companies;
12) failure to effectively manage newly acquired businesses and the
cost related to such integration;
13) adverse results in litigation matters;
14) inability to attract and retain experienced and qualifi ed personnel;
15) failure to maintain the Company’s days sales outstanding levels;
16) decrease in credit ratings by Standard & Poor’s and/or Moody’s;
17) failure to develop or acquire licenses for new or improved
technologies, or if customers use new technologies to perform
their own tests;
18) inability to commercialize newly licensed tests or technologies
or to obtain appropriate coverage or reimbursement for such
tests, which could result in impairment in the value of certain
capitalized licensing costs;
19) inability to obtain and maintain adequate patent and other
proprietary rights for protection of the Company’s products
and services and successfully enforce the Company’s
proprietary rights;
20) the scope, validity and enforceability of patents and other
proprietary rights held by third parties which might have an
impact on the Company’s ability to develop, perform, or market
the Company’s tests or operate its business;
21) failure in the Company’s information technology systems
resulting in an increase in testing turnaround time or billing
processes or the failure to meet future regulatory or customer
information technology and connectivity requirements;
22) failure of the Company’s fi nancial information systems resulting
in failure to meet required fi nancial reporting deadlines;
23) failure of the Company’s disaster recovery plans to provide
adequate protection against the interruption of business and/or
to permit the recovery of business operations;
24) business interruption or other impact on the business due to
adverse weather (including hurricanes), fi res and/or other
natural disasters and terrorism or other criminal acts;
25) liabilities that result from the inability to comply with new
corporate governance requirements; and
26) signifi cant deterioration in the economy could negatively
impact the Company’s testing volumes, cash collections and
the availability of credit.
Laboratory Corporation of America