Pfizer 2015 Annual Report Download - page 12
Download and view the complete annual report
Please find page 12 of the 2015 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
2015 Financial Report
11
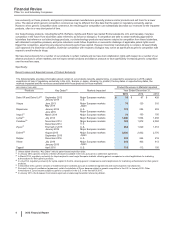
Phase 1 trials. Under the terms of the agreement, in the fourth quarter of 2014, we made an upfront payment of $850 million to Merck
KGaA and Merck KGaA is eligible to receive regulatory and commercial milestone payments of up to approximately $2.0 billion. Both
companies will jointly fund all development and commercialization costs, and split equally any profits generated from selling any anti-PD-
L1 or anti-PD-1 products from this collaboration. Also, as part of the agreement, we gave Merck KGaA certain co-promotion rights for
Xalkori in the U.S. and several other key markets, and co-promotion activities were initiated in key select markets in 2015. In 2014, we
recorded $1.2 billion of Research and development expenses associated with this collaborative arrangement, composed of the $850
million upfront cash payment as well as an additional amount of $309 million, reflecting the estimated fair value of the co-promotion rights
given to Merck KGaA.
• Acquisition of InnoPharma, Inc. (InnoPharma)––On September 24, 2014, we completed our acquisition of InnoPharma, a privately-held
pharmaceutical development company, for an upfront cash payment of $225 million and contingent consideration of up to $135 million.
• License from Cellectis SA (Cellectis)––In June 2014, we entered into a global arrangement with Cellectis to develop Chimeric Antigen
Receptor T-cell immunotherapies in the field of oncology directed at select cellular surface antigen targets. In August 2014, in connection
with this licensing agreement, we made an upfront payment of $80 million to Cellectis, which was recorded in Research and development
expenses. We will also fund research and development costs associated with 15 Pfizer-selected targets and, for the benefit of Cellectis, a
portion of the R&D costs associated with four Cellectis-selected targets within the arrangement. Cellectis is eligible to receive
development, regulatory and commercial milestone payments of up to $185 million per product that results from the Pfizer-selected
targets. Cellectis is also eligible to receive tiered royalties on net sales of any products that are commercialized by Pfizer.
• Investment in ViiV Healthcare Limited (ViiV)––On January 21, 2014, the European Commission approved Tivicay (dolutegravir), a product
for the treatment of HIV-1 infection, developed by ViiV, an equity-method investee. This approval, in accordance with the agreement
between GlaxoSmithKline plc and Pfizer, triggered a reduction in our equity interest in ViiV from 12.6% to 11.7% and an increase in
GlaxoSmithKline plc’s equity interest in ViiV from 77.4% to 78.3%, effective April 1, 2014. As a result, in 2014, we recognized a loss of
approximately $30 million in Other (income)/deductions––net. We account for our investment in ViiV under the equity method due to the
significant influence that we continue to have through our board representation and minority veto rights.
• Collaboration with Eli Lilly & Company (Lilly)––In October 2013, we entered into a collaboration agreement with Lilly to jointly develop and
globally commercialize Pfizer’s tanezumab, which provides that Pfizer and Lilly will equally share product-development expenses as well
as potential revenues and certain product-related costs. Following the decision by the FDA in March 2015 to lift the partial clinical hold on
the tanezumab development program, we received a $200 million upfront payment from Lilly in accordance with the collaboration
agreement between Pfizer and Lilly, which is recorded as deferred revenue in our consolidated balance sheet and is being recognized into
Other (income)/deductions––net over a multi-year period beginning in the second quarter of 2015. Pfizer and Lilly resumed the Phase 3
chronic pain program for tanezumab in July 2015, which will consist of six studies in approximately 7,000 patients across osteoarthritis,
chronic low back pain and cancer pain. Under the collaboration agreement with Lilly, we are eligible to receive additional payments from
Lilly upon the achievement of specified regulatory and commercial milestones.
• Divestiture of Zoetis––On June 24, 2013, we completed the full disposition of Zoetis. The full disposition was completed through a series of
steps, including, in the first quarter of 2013, the formation of Zoetis and an initial public offering (IPO) of an approximate 19.8% interest in
Zoetis and, in the second quarter of 2013, an exchange offer for the remaining 80.2% interest.
• Collaboration with Merck & Co., Inc. (Merck)––In April 2013, we announced that we entered into a worldwide (except Japan) collaboration
agreement with Merck for the development and commercialization of Pfizer’s ertugliflozin (PF-04971729), an investigational oral sodium
glucose cotransporter (SGLT2) inhibitor currently in Phase 3 development for the treatment of type 2 diabetes.
• Investment in Hisun Pfizer Pharmaceuticals Company Limited (Hisun Pfizer)––On September 6, 2012, we and Zhejiang Hisun
Pharmaceuticals Co., Ltd. (Hisun), a leading pharmaceutical company in China, formed a new company, Hisun Pfizer, to develop,
manufacture, market and sell pharmaceutical products, primarily branded generic products, predominately in China. In the first quarter of
2013, we and Hisun contributed certain assets to Hisun Pfizer. Hisun Pfizer is 49% owned by Pfizer and 51% owned by Hisun. Our
contributions constituted a business, as defined by U.S. GAAP, and in 2013, we recognized a pre-tax gain of approximately $459 million in
Other (income)/deductions––net. In the third quarter of 2015, we determined that we had an other-than-temporary decline in value of our
equity-method investment in Hisun Pfizer, and, therefore, in 2015, we recognized a loss of $463 million in Other (income)/deductions––net.
The decline in value resulted from lower expectations as to the future cash flows to be generated by Hisun Pfizer, as a result of lower than
expected recent performance, increased competition, a slowdown in the China economy in relation to their products, as well as changes in
the regulatory environment.
• License of Nexium OTC Rights––In August 2012, we entered into an agreement with AstraZeneca PLC (AstraZeneca) for the exclusive,
global, over-the-counter (OTC) rights for Nexium, a leading prescription drug approved to treat the symptoms of gastroesophageal reflux
disease. In connection with this Consumer Healthcare licensing agreement, we made an upfront payment of $250 million to AstraZeneca,
which was recorded in Research and development expenses when incurred. On May 27, 2014, we launched Nexium 24HR in the U.S.,
and on July 11, 2014, we paid AstraZeneca a related $200 million product launch milestone payment. On August 1, 2014, we launched
Nexium Control in Europe, and on September 15, 2014, we paid AstraZeneca a related $50 million product launch milestone payment.
These post-approval milestone payments were recorded in Identifiable intangible assets, less accumulated amortization and are being
amortized over the estimated useful life of the Nexium brand. Included in Other current liabilities at December 31, 2015 are accrued
milestone payments to AstraZeneca of $93 million. AstraZeneca is eligible to receive additional milestone payments of up to $200 million,
based on the level of worldwide sales as well as quarterly royalty payments based on worldwide sales.