Pfizer 2015 Annual Report Download - page 5
Download and view the complete annual report
Please find page 5 of the 2015 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
4
2015 Financial Report
lose exclusivity on these products, and generic pharmaceutical manufacturers generally produce similar products and sell them for a lower
price. The date at which generic competition commences may be different from the date that the patent or regulatory exclusivity expires.
However, when generic competition does commence, the resulting price competition can substantially decrease our revenues for the impacted
products, often in a very short period of time.
Our biotechnology products, including BeneFIX, ReFacto, Xyntha and Enbrel (we market Enbrel outside the U.S. and Canada), may face
competition in the future from biosimilars (also referred to as follow-on biologics). If competitors are able to obtain marketing approval for
biosimilars that reference our biotechnology products, our biotechnology products may become subject to competition from these biosimilars,
with attendant competitive pressure, and price reductions could follow. Expiration or successful challenge of applicable patent rights could
trigger this competition, assuming any relevant exclusivity period has expired. However, biosimilar manufacturing is complex. At least initially
upon approval of a biosimilar competitor, biosimilar competition with respect to biologics may not be as significant as generic competition with
respect to small molecule drugs.
We have lost exclusivity for a number of our products in certain markets and we have lost collaboration rights with respect to a number of our
alliance products in certain markets, and we expect certain products and alliance products to face significantly increased generic competition
over the next few years.
Specifically:
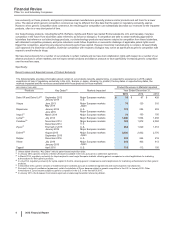
Recent Losses and Expected Losses of Product Exclusivity
The following table provides information about certain of our products recently experiencing, or expected to experience in 2016, patent
expirations or loss of regulatory exclusivity in the U.S., Europe or Japan, showing, by product, the key dates or expected key dates, the
markets impacted and the revenues associated with those products in those markets:
(MILLIONS OF DOLLARS) Product Revenues in Markets Impacted
Products Key Dates(a) Markets Impacted Year Ended December 31,
2015 2014 2013
Detrol IR and Detrol LA(b) September 2012
January 2014
Major European markets
U.S. $35$87$
428
Viagra June 2013
May 2014
Major European markets
Japan 76 120 310
Rapamune January 2014
June 2015
U.S.
Major European markets 129 254 253
Inspra(c) March 2014 Major European markets 74 160 150
Lyrica(d) July 2014 Major European markets 1,048 1,634 1,458
Celebrex(e) November 2014
December 2014
Major European markets
U.S. 189 1,872 2,084
Zyvox(f) First half of 2015
January 2016
U.S.
Major European markets 564 1,020 1,013
Enbrel(g) August 2015
September 2015
Major European markets
Japan 2,402 2,832 2,776
Relpax December 2016 U.S. 233 244 218
Vfend July 2016
January 2016
Major European markets
Japan 349 403 413
Tygacil April 2016 U.S. 110 112 150
(a) Unless stated otherwise, “Key Dates” indicate patent-based expiration dates.
(b) In January 2014, generic versions of Detrol LA became available in the U.S. pursuant to a settlement agreement.
(c) In March 2014, regulatory exclusivity for Inspra expired in most major European markets, allowing generic companies to submit applications for marketing
authorizations for their generic products.
(d) In July 2014, regulatory exclusivity for Lyrica expired in the EU, allowing generic companies to submit applications for marketing authorizations for their generic
products.
(e) In December 2014, generic versions of Celebrex became available pursuant to settlement agreements with several generic manufacturers.
(f) Pursuant to terms of a settlement agreement, certain formulations of Zyvox became subject to generic competition in the U.S. in January 2015. Other
formulations of Zyvox became subject to generic competition in the U.S. in the first half of 2015.
(g) In January 2016, the European Commission approved an etanercept biosimilar referencing Enbrel.