Pfizer 2015 Annual Report Download - page 7
Download and view the complete annual report
Please find page 7 of the 2015 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
6
2015 Financial Report
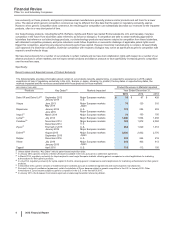
Impacts on our 2014 Results
We recorded the following amounts in 2014 as a result of the U.S. Healthcare Legislation:
• $631 million recorded as a reduction to Revenues, related to the higher, extended and expanded rebate provisions and the Medicare
“coverage gap” discount provision; and
• $362 million recorded in Selling, informational and administrative expenses, related to the fee payable to the federal government. 2014
included a $215 million charge to account for an additional year of the non-tax deductible Branded Prescription Drug Fee in accordance
with final regulations issued in the third quarter of 2014 by the IRS. The amount in 2014 also reflected a favorable true-up associated with
the final 2013 invoice received from the federal government, which reflected a lower share than that of the initial 2013 invoice.
The final regulations issued by the IRS did not change the payment schedule for the Branded Prescription Drug Fee; accordingly there was no
cash flow impact in 2014 from the $215 million charge.
Impacts on our 2013 Results
We recorded the following amounts in 2013 as a result of the U.S. Healthcare Legislation:
• $458 million recorded as a reduction to Revenues, related to the higher, extended and expanded rebate provisions and the Medicare
“coverage gap” discount provision; and
• $280 million recorded in Selling, informational and administrative expenses, related to the fee payable to the federal government.
Regulatory Environment/Pricing and Access––Government and Other Payer Group Pressures
Governments, managed care organizations and other payer groups continue to seek increasing discounts on our products through a variety of
means, such as leveraging their purchasing power, implementing price controls, and demanding price cuts (directly or by rebate actions). In
Europe, Japan, China, Canada, South Korea and some other international markets, governments provide healthcare at low direct cost to
patients and regulate pharmaceutical prices or patient reimbursement levels to control costs for the government-sponsored healthcare system.
In the U.S., a primary government activity with implications for pharmaceutical pricing is deficit reduction. Any significant spending reductions
affecting Medicare, Medicaid or other publicly funded or subsidized health programs that may be implemented, and/or any significant
additional taxes or fees that may be imposed on us, as part of any broad deficit-reduction effort could have an adverse impact on our results of
operations.
Additionally, policy efforts designed specifically to reduce patient out-of-pocket costs for medicines could result in new mandatory rebates and
discounts or other pricing restrictions. A number of the candidates for the 2016 U.S. presidential elections have introduced such policy
proposals, and a November 2015 U.S. Department of Health and Human Services forum dedicated to drug pricing could lead to further
proposals. We believe medicines are the most efficient and effective use of healthcare dollars based on the value they deliver to the overall
healthcare system. We continue to work with stakeholders in an effort to ensure access to medicines within an efficient and affordable
healthcare system. In addition, certain regulatory changes to be implemented in 2016 may affect Pfizer's obligations under the Medicaid drug
rebate program, but the impact of those changes is not yet known.
The ACA, which expanded the role of the U.S. government as a healthcare payer, is accelerating changes in the U.S. healthcare marketplace,
and the potential for additional pricing and access pressures continues to be significant. Many of these developments may impact drug
utilization, in particular branded drug utilization. Some employers, seeking to avoid the tax on high-cost health insurance in the ACA originally
to be imposed in 2018 (now to be imposed in 2020, per the terms of the fiscal year 2016 omnibus appropriations legislation), are already
scaling back healthcare benefits. Some health plans and pharmacy benefit managers are seeking greater pricing predictability from
pharmaceutical manufacturers in contractual negotiations. Other health plans and pharmacy benefit managers are increasing their focus on
spending on specialty medicines by implementing co-insurance in place of a flat co-payment. Because co-insurance passes on a percentage
of a drug’s cost to the patient, this shift has the potential to significantly increase patient out-of-pocket costs.
Overall, there is increasing pressure on U.S. providers to deliver healthcare at a lower cost and to ensure that those expenditures deliver
demonstrated value in terms of health outcomes. Longer term, we are seeing a shift in focus away from fee-for-service payments towards
outcomes-based payments and risk-sharing arrangements that reward providers for cost reductions. These new payment models can, at
times, lead to lower prices for, and restricted access to, new medicines. At the same time, these models can also expand utilization by
encouraging physicians to screen, diagnose and focus on outcomes.
In response to the evolving U.S. and global healthcare spending landscape, we are continuing to work with health authorities, health
technology assessment and quality measurement bodies and major U.S. payers throughout the product-development process to better
understand how these entities value our compounds and products. Further, we are seeking to develop stronger internal capabilities focused on
demonstrating the value of the medicines that we discover or develop, register and manufacture, by recognizing patterns of usage of our
medicines and competitor medicines along with patterns of healthcare costs.
Regulatory Environment––Pipeline Productivity
The discovery and development of safe, effective new products, as well as the development of additional uses for existing products, are
necessary for the continued strength of our businesses. We have encountered increasing regulatory scrutiny of drug safety and efficacy, even
as we continue to gather safety and other data on our products, before and after the products have been launched. Our product lines must be
replenished over time in order to offset revenue losses when products lose their market exclusivity, as well as to provide for earnings growth.
We devote considerable resources to R&D activities. These activities involve a high degree of risk and cost and may take many years, and