Medtronic 2008 Annual Report Download - page 23
Download and view the complete annual report
Please find page 23 of the 2008 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
benefits in the “Special, Restructuring, Certain Litigation, and IPR&D
Charges, and Certain Tax Adjustments” section of this management’s
discussion and analysis. The fiscal year 2008 special, restructuring, IPR&D
and certain litigation charges more than offset the positive earnings
growth from core operations.
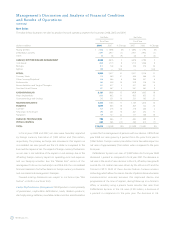
Net Sales
Fiscal Year
(dollars in millions) 2008 2007 % Change
Cardiac Rhythm Disease Management $ 4,963 $ 4,876 2%
Spinal 2,982 2,417 23
CardioVascular 2,131 1,909 12
Neuromodulation 1,311 1,183 11
Diabetes 1,019 863 18
Surgical Technologies 780 666 17
Physio-Control 329 385 (15)
Total Net Sales
$ 13,515
$ 12,299
10
%
Net sales in fiscal year 2008 were $13.515 billion, an increase of
10 percent from the prior fiscal year. Foreign currency translation had a
favorable impact of $400 million on net sales when compared to fiscal
year 2007. The net sales increase in the current fiscal year was fortified
by the addition of Kyphon to our Spinal business and led by organic
double digit sales growth in the CardioVascular, Diabetes,
Neuromodulation and Surgical Technologies businesses. Growth
outside the United States (U.S.) was also especially strong, where six of
our seven operating segments had strong double digit growth rates.
See our discussion in the “Net Sales” section of this management’s
discussion and analysis for more information on the results of our
significant operating segments.
We remain committed to our Mission of developing lifesaving and
life enhancing therapies to alleviate pain, restore health and extend life.
The diversity and depth of our current product offerings enable us to
provide medical therapies to patients worldwide. We will work to
improve patient access through well planned studies, which show the
cost-effectiveness of our therapies and our alliance with patients,
clinicians, regulators and reimbursement agencies. Our investments in
research and development, strategic acquisitions, expanded clinical
trials and infrastructure provide the foundation for our growth. We are
confident in our ability to drive long-term shareholder value using the
principles of our Mission, our strong product pipelines and continued
commitment to innovative research and development.
Other Matters
On October 15, 2007, we announced the voluntary suspension of
worldwide distribution of Sprint Fidelis (Fidelis) leads because of the
potential for lead fractures at higher than anticipated rates. Leads are
sophisticated “wires” that connect an electronic pulse generator to the
heart and are the pathway for therapy delivery between the device and
heart. The Fidelis leads are applicable to therapy delivery in defibrillators
only, including implantable cardioverter defibrillators (ICDs) and cardiac
resynchronization therapy-defibrillators (CRT-Ds). The decision to
voluntarily suspend the worldwide distribution of the Fidelis lead was
based on a variety of factors that, when viewed together, indicated a
voluntary suspension was the appropriate action. Based on Medtronic’s
extensive performance data, Fidelis lead viability was trending lower
than Medtronic’s Sprint Quattro (Quattro) lead at 30 months after
implant (97.7 percent Fidelis vs. 99.1 percent Quattro). This difference
was not considered statistically significant; however, if the current lead
fracture rates remain constant, it could become significant over time.
We believed that given this performance trend, this suspension of
worldwide distribution was in the patients’ best interests.
When we ceased selling Fidelis leads and asked customers to return
their unused product, Fidelis leads represented approximately
75 percent of our high power lead manufacturing output with our
Quattro leads representing the other 25 percent. We successfully
transitioned our manufacturing back to the production of Quattro leads
and, by the end of the third quarter of fiscal year 2008, had re-established
sufficient internal inventory levels to meet customer demand. Even
though we quickly re-established our internal inventory levels, we believe
we missed selling opportunities in the second, third and fourth quarters of
fiscal year 2008 due to the voluntary suspension of worldwide distribution
of Fidelis leads, the lack of a single coil lead and the lack of an approved
lead in Japan for most of the third quarter of fiscal year 2008. In January
2008, we were able to begin selling our Quattro lead in Japan after
receiving both regulatory and reimbursement approvals.
On December 4, 2006, we announced our intention to pursue a
spin-off of Physio-Control into an independent, publicly traded
company. Physio-Control is our wholly-owned subsidiary that offers
external defibrillators, emergency response systems, data management
solutions and support services used by hospitals and emergency
response personnel. However, shortly thereafter, in January 2007, we
announced a voluntary suspension of U.S. shipments of Physio-Control
products manufactured at our facility in Redmond, Washington in order
to address quality system issues. In the months following the suspension
of U.S. shipments, we worked diligently with the U.S. Food and Drug
Administration (FDA) to address the quality system issues and resumed
limited shipments to critical need customers. As a result of the work
performed to date, on April 28, 2008, we announced that we had
19Medtronic, Inc.