Medtronic 2008 Annual Report Download - page 28
Download and view the complete annual report
Please find page 28 of the 2008 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
risk of sudden cardiac arrest. The Secura ICD and the Consulta
CRT-D, the portfolio’s first ICD and CRT-D devices, are expected to
be commercially available in the coming months. Vision 3D is our
first generation device with a common platform across ICDs,
CRT-Ds and pacing systems. Additionally, these products provide
enhanced follow-up and automaticity features and create
meaningful manufacturing synergies. We will continue to develop
our industry leading product portfolio to meet the medical needs
of our patients.
• The future acceptance of our single coil Quattro lead, which we
expect to launch in markets around the world in the first quarter
of fiscal year 2009. Some physicians prefer a single coil lead,
particularly physicians in certain Western European countries. We
believe the future availability of this product will help us to further
recover from the impact of the Fidelis lead issue.
• Continued acceptance of the Adapta family of pacemakers,
including the Adapta, Versa and Sensia models.
• Continued expansion of the Medtronic CareLink Service, available
on both the Pacing and Defibrillator platforms in the U.S., Canada
and Western Europe, and beginning in the fourth quarter of fiscal
year 2008, on a pilot basis in Japan and Australia. We believe
Medtronic CareLink Service continues to drive physician preference
for our products.
• The future launch and acceptance of the EnRhythm MRI SureScan
pacing system (EnRhythm MRI). EnRhythm MRI will be the first
pacemaker system to be developed and tested specifically for safe
use in Magnetic Resonance Imaging (MRI) machines under specified
scanning conditions. EnRhythm MRI is designed to address and
mitigate interactions between the pacing system and the magnetic
resonance environment.
Our growth in CRDM has been and will continue to be contingent
upon continued market growth and on our ability to maintain our
market position.
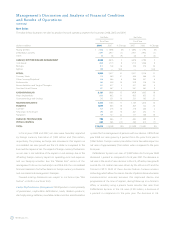
Spinal Spinal products include thoracolumbar, cervical and interbody
spinal devices, bone growth substitutes and devices for vertebral
compression fractures and spinal stenosis. Spinal net sales for fiscal year
2008 increased by 23 percent from the prior fiscal year to $2.982 billion.
Foreign currency translation had a favorable impact on net sales of
$44 million when compared to the prior fiscal year. The growth in fiscal
year 2008 was primarily driven by the November 2, 2007 close of the
acquisition of Kyphon, which generated revenue of $298 million during
the fiscal year.
Core Spinal net sales for fiscal year 2008 were $1.869 billion, an
increase of 9 percent from the prior fiscal year. Growth in the period was
primarily based on continued acceptance of our products for the
thoracolumbar and cervical sections of the spine. Net sales in fiscal year
2008 were hampered by the trend of small companies increasing their
presence and placing pressure on the Core Spinal market. Today, there
are over 200 small physician owned companies competing in the
marketplace. Thoracolumbar net sales growth for fiscal year 2008 was
driven by net sales of the CD HORIZON LEGACY family of products
(CD HORIZON) and the CAPSTONE Vertebral Body Spacer (CAPSTONE)
outside the U.S., net sales of the VERTE-STACK CRESCENT Vertebral Body
Spacer (CRESCENT) for thoracolumbar stabilization in the U.S. and
worldwide net sales growth of the Lumbar Dynamic platform of
products. CD HORIZON is the most comprehensive system on the
market today, and is designed to provide procedural solutions for
degenerative, deformity or trauma applications using color coded
implants and ergonomic instrumentation. The CAPSTONE and
CRESCENT are minimal access devices and techniques designed to
replace and restore vertebral height in the thoracolumbar spine. The
growth of our Lumbar Dynamic platform of products, which allow
some range in motion as compared to our fixed stabilization devices,
was driven by demand for our PEEK Rod System in the U.S. and
DIAM System outside the U.S. The growth in net sales in our cervical
products during the fiscal year was led by the continued acceptance of
the VERTEX Max Reconstruction System for cervical stabilization outside
the U.S.
Biologics net sales for fiscal year 2008 increased 16 percent from the
prior fiscal year to $815 million. This increase was primarily driven by
continued strong acceptance of INFUSE Bone Graft in the U.S. INFUSE
Bone Graft contains a recombinant human bone morphogenetic
protein, or rhBMP-2, that induces the body to grow its own bone,
eliminating the need for a painful second surgery to harvest bone from
elsewhere in the body. In addition to FDA approval for use of INFUSE
Bone Graft for spinal fusion, we received FDA approval to use INFUSE
Bone Graft for the treatment of certain types of acute, open fractures
of the tibial shaft in fiscal year 2005, and for certain oral maxillofacial
and dental regenerative bone grafting procedures late in fiscal year
2007. Additionally, although on a smaller base, we have continued to
experience strong fiscal year 2008 growth in the sales of InductOs Bone
Graft, the outside the U.S. equivalent of INFUSE Bone Graft.
Kyphon, which was acquired on November 2, 2007, had net sales of
$298 million for fiscal year 2008 that were driven by continued
acceptance of balloon kyphoplasty procedures for treating vertebral
compression fractures and acceptance of Kyphon’s interspinous
Management’s Discussion and Analysis of Financial Condition
and Results of Operations
(continued)
24 Medtronic, Inc.