Medtronic 2008 Annual Report Download - page 32
Download and view the complete annual report
Please find page 32 of the 2008 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Looking ahead, we expect our Neuromodulation operating segment
should benefit from the following:
• Continued acceptance of RestoreULTRA, our next generation
rechargeable neurostimulator with advanced programming
capabilities and smaller device size, which was launched in the
fourth quarter of fiscal year 2008. RestoreULTRA is the smallest and
thinnest 16-electrode rechargeable neurostimulator on the market
and offers an innovative patient programmer that gives patients
the ability to customize their pain control.
• Continued acceptance of our surgical lead, the Specify 5-6-5 with
Durable Electrode Technology, which was launched in the first quarter
of fiscal year 2008. The Specify 5-6-5 surgical lead offers exclusive
advantages and electrode programming patterns when used with
our neurostimulators. Additionally, we anticipate the launch of the
Specify 2x8 surgical lead in the first half of fiscal year 2009.
• Continued acceptance of our Activa DBS Therapy for the treatment
of common movement disorders. We continue to educate
neurologists and the patient population on the benefits that our
Activa DBS Therapy offers them. Additionally, we look forward to
the anticipated launch of Activa PC and RC, our next generation
neurostimulators. Activa PC is a primary cell and Activa RC will be
the therapy’s first rechargeable device. We anticipate launch of
Activa RC in the second half of fiscal year 2009.
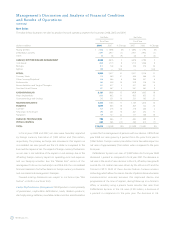
Diabetes Diabetes products consist of external insulin pumps and
related consumables, continuous glucose monitoring systems and
subcutaneous glucose sensors. Diabetes net sales in fiscal year 2008
increased 18 percent over the prior fiscal year to $1.019 billion. Foreign
currency translation had a favorable impact of $29 million on net sales
when compared to the prior fiscal year.
External pump sales for fiscal year 2008 were $448 million,
representing growth of 15 percent over the prior fiscal year. This increase
reflects strong worldwide market acceptance of the Paradigm REAL-Time
sensor-augmented pump system that integrates continuous glucose
monitoring and insulin pump functionality. The sales increase of
41 percent outside the U.S. was especially strong, driven by growth in
the markets in which we recently launched the Paradigm Real-Time
system. The strong growth outside the U.S. was offset by slowed growth
in the U.S., as we experienced a modest slowdown in replacement
business given the timing of upgrades to our latest technology. Net
sales of Consumables, including glucose sensors and other monitoring
equipment, during fiscal year 2008 were $571 million, an increase of
20 percent. Net sales of infusion sets outside the U.S., in correlation with
our strong pump growth, fueled the growth in Consumables.
Diabetes net sales in fiscal year 2007 increased 20 percent over the
prior fiscal year to $863 million. Foreign currency translation had a
favorable impact of $13 million on net sales when compared to the prior
fiscal year.
External pump sales for fiscal year 2007 were $389 million, representing
growth of 32 percent over the prior fiscal year. This increase reflects
strong worldwide market acceptance of the Paradigm REAL-Time
sensor-augmented pump system. Net sales of Consumables, including
glucose monitoring system and sensor products and other equipment,
during fiscal year 2007 were $474 million, an increase of 11 percent.
Looking ahead, we expect our Diabetes operating segment should
benefit from the following:
• Continued acceptance from both physicians and patients of the
Paradigm REAL-Time sensor-augmented pump system, which
integrates continuous glucose monitoring and insulin pump
functionality.
• Continued acceptance of the Guardian REAL-Time System, our
personal-use Continuous Glucose Monitoring System (CGMS) for
diabetes management. The Guardian REAL-Time System is a
stand-alone glucose monitoring system that provides patients with
real-time glucose trend graphs and predictive alarms informing
them when their glucose levels become too high or too low,
enabling better management of diabetes.
• Future acceptance and customer preference for Medtronic products
due to the alliances with LifeScan, Inc. (LifeScan), a Johnson &
Johnson company, and Bayer Diabetes Care (Bayer), a member of
the Bayer group, which we announced on August 21, 2007. The
alliances reached with Lifescan (for the U.S. market) and Bayer (for
markets outside the U.S.) provide for the distribution and marketing
of blood glucose meters that communicate with Medtronic’s insulin
pumps. These alliances provide our customers an integrated
solution for managing diabetes, thereby improving the quality of
life and ease of use. We launched our co-developed blood glucose
meters with Bayer and LifeScan in February 2008 and April 2008,
respectively.
• Improved reimbursement for insulin pumps in certain international
markets and for continuous glucose monitoring in both the U.S.
and certain international markets.
• Completion of the first user evaluation of a partially-closed loop
system in the United Kingdom and the Netherlands. The study
Management’s Discussion and Analysis of Financial Condition
and Results of Operations
(continued)
28 Medtronic, Inc.