Medtronic 2008 Annual Report Download - page 36
Download and view the complete annual report
Please find page 36 of the 2008 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
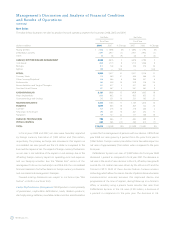
This global realignment initiative will result in charges being
recognized in both the fourth quarter of fiscal year 2008 and the first
quarter of fiscal year 2009, and we expect that when complete, will
eliminate approximately 1,100 positions. Restructuring charges were
recognized in the fourth quarter of fiscal year 2008 for standard
severance benefits to be provided to impacted positions identified prior
to the close of the fiscal year. In the first quarter of fiscal year 2009 we
will recognize additional restructuring charges associated with
(i) enhanced severance benefits for positions, identified in the fourth
quarter of fiscal year 2008, and (ii) standard and enhanced severance
benefits provided for positions that were identified in the first quarter
of fiscal year 2009. These incremental costs were not accrued in fiscal
year 2008 because either the enhanced benefits had not yet been
communicated to the impacted employees or the positions for
elimination had not yet been identified. We anticipate that the additional
expense that we will recognize in the first quarter of fiscal year 2009
related to the global realignment initiative will be in the range of
$80 million to $105 million.
Of the 1,100 positions that will be eliminated as part of this initiative,
560 positions were identified for elimination in the fourth quarter of
fiscal year 2008 and will be achieved through voluntary and involuntary
separation. Of these 560 positions identified, the majority will be
eliminated in fiscal year 2009. The restructuring initiatives related to the
560 employees identified in the fourth quarter of fiscal year 2008 are
scheduled to be completed by the end of fiscal year 2009, and are expected
to produce annualized operating savings of approximately $69 million.
These savings will arise mostly from reduced compensation expense. See
Note 3 to the consolidated financial statements for further discussion.
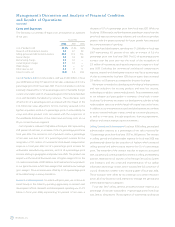
Fiscal Year 2007 Initiative In fiscal year 2007, we recorded a $36 million
restructuring charge, which consisted of employee termination costs
of $28 million and asset write-downs of $8 million. These initiatives were
designed to drive manufacturing efficiencies in our CardioVascular
business, downsize our Physio-Control business due to our voluntary
suspension of U.S. shipments and rebalance resources within our CRDM
business in response to market dynamics. The employee termination
costs consist of severance and the associated costs of continued
medical benefits, and outplacement services. The asset write-downs
consist of a $5 million charge for inventory write-downs and a $3 million
charge for non-inventory asset write-downs. The inventory and asset
write-downs were recorded within cost of products sold in the
consolidated statement of earnings.
As a continuation of our fiscal year 2007 initiatives, in the first quarter
of fiscal year 2008 we incurred $14 million of incremental restructuring
charges associated with compensation provided to employees whose
employment terminated with the Company in the first quarter of fiscal
year 2008. These incremental costs were not accrued in fiscal year 2007
because these benefits had not yet been communicated to the
impacted employees. Included in the total $14 million restructuring
charge is $4 million of incremental defined benefit pension and
post-retirement related expense for those employees who accepted
early retirement packages. For further discussion on the incremental
defined benefit pension and post-retirement related expense, see
Note 13 to the consolidated financial statements.
When the restructuring initiative began in fiscal year 2007, we
identified approximately 900 positions for elimination which were
achieved through early retirement packages offered to employees,
voluntary separation and involuntary separation, as necessary. As of
April 25, 2008, the initiatives begun in the fourth quarter of fiscal year
2007 were substantially complete. This restructuring initiative produced
annualized operating savings of approximately $125 million mostly from
reduced compensation expense. See Note 3 to the consolidated
financial statements for further discussion.
There were no restructuring charges in fiscal year 2006.
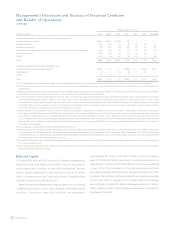
Certain Litigation Charges We classify material litigation reserves
recognized as certain litigation charges.
During fiscal year 2008, we incurred certain litigation charges of
$366 million. Of that amount, $123 million relates to the settlement of
certain lawsuits relating to the Marquis line of ICDs and CRT-Ds that
were subject to a field action announced on February 10, 2005. The
remainder of the charge, $243 million, relates to an estimated reserve
established for litigation with Cordis Corporation, a subsidiary of
Johnson & Johnson. The Cordis litigation originated in October 1997
and pertains to a patent infringement claim on a previous generation
of bare metal stents that are no longer on the market. We believe an
unfavorable outcome in the Cordis matter is probable. In accordance
with SFAS No. 5, we have recorded a $243 million reserve for estimated
damages in this matter. See Notes 2 and 15 to the consolidated financial
statements for further discussion of these certain litigation charges. In
May 2008, we paid substantially all of the settlement for certain lawsuits
relating to the Marquis line of ICDs and CRT-Ds.
During fiscal year 2007, we recorded a certain litigation charge of
$40 million related to a settlement agreement with the U.S. Department
of Justice which requires the government to obtain dismissal of two qui
tam civil suits pending against us, and is conditioned upon such
dismissal being obtained. The two suits were based upon allegations
about certain sales and marketing practices in the Spinal business.
The settlement agreement reflects our assertion that the Company
Management’s Discussion and Analysis of Financial Condition
and Results of Operations
(continued)
32 Medtronic, Inc.