Medtronic 2011 Annual Report Download - page 103
Download and view the complete annual report
Please find page 103 of the 2011 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
99
Medtronic, Inc.
Investor Information
Annual Meeting
The annual meeting of Medtronic shareholders will take place
on Thursday, August 25, 2011, beginning at 10:30 a.m. (Central
Daylight Time) at Medtronic’s world headquarters, 710 Medtronic
Parkway, Minneapolis (Fridley), Minnesota.
Investor Information
Shareholders, securities analysts, and investors seeking more
information about the Company can access the following
information via the Internet at www.medtronic.com:
• News releases describing significant Company events, and sales
and earnings results for each quarter and the fiscal year.
• Form 10-K Annual; Form 10-Q Quarterly; and Forms 3, 4, and 5,
Reports to the Securities and Exchange Commission describing
Medtronic’s business, and financial condition and insider trading.
The information above may also be obtained upon request from
the Medtronic Investor Relations Department, 710 Medtronic
Parkway, Minneapolis, Minnesota 55432, USA.
Stock Exchange Listing
New York Stock Exchange (symbol: MDT)
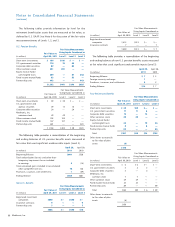
Price Range of Medtronic Common Stock
Fiscal Quarter
First
Quarter
Second
Quarter
Third
Quarter
Fourth
Quarter
2011 High $44.13 $37.90 $38.51 $41.86
2011 Low 36.03 31.21 33.53 36.67
2010 High $35.83 $39.06 $46.03 $45.81
2010 Low 29.96 35.58 35.99 41.67
Prices are closing quotations. On June 27, 2011, there were
approximately 49,950 shareholders of record of the Company’s
common stock. The regular quarterly cash dividend was 22.50
cents per share for fiscal year 2011 and 20.50 cents per share for
fiscal year 2010.
Stock Transfer Agent and Registrar
Wells Fargo Shareowner ServicesSM acts as transfer agent and
registrar, dividend paying agent, and direct stock purchase plan
agent for Medtronic and maintains all shareholder records for the
Company. If you are a registered shareholder, you may access
your account information online at www.shareowneronline.com.
If you have questions regarding the Medtronic stock you own,
stock transfers, address or name changes, direct deposit of
dividends, lost dividend checks, lost stock certificates, or duplicate
mailings, please contact Wells Fargo Shareowner ServicesSM by
writing or calling:
Wells Fargo Bank, N. A.
Shareowner Services
161 North Concord Exchange
South St. Paul, MN 55075 USA
Telephone: 888-648-8154 or 651-450-4064
Fax: 651-450-4033
www.wellsfargo.com/shareownerservices
Direct Stock Purchase Plan
Medtronic’s transfer agent, Wells Fargo Shareowner ServicesSM,
administers the direct stock purchase plan, which is called the
Shareowner Service Plus PlanSM. Features of this plan include
direct stock purchase and reinvestment of dividends to purchase
whole or fractional shares of Medtronic stock. All registered
shareholders and potential investors may participate.
To request information on the Shareowner Service Plus PlanSM,
or to enroll in the plan, contact Wells Fargo Shareowner ServicesSM
at 888-648-8154 or 651-450-4064. You may also enroll on the
Internet by visiting www.shareowneronline.com and selecting
“Direct Purchase Plan.”
Independent Registered Public Accounting Firm
PricewaterhouseCoopers LLP, Minneapolis, MN
Diversity
Medtronic is committed to creating and maintaining a workplace
that reflects the diversity of our customers, patients and the
communities we serve. Consistent with our Mission, Medtronic
“recognizes the personal worth of employees” and seeks to
provide a work environment where individual differences are
valued and respected and opportunities for growth and career
success are based on individual merit.
Officer Certifications
Medtronic has filed as exhibits to its Annual Report on Form 10-K
for the fiscal year ended April 29, 2011, the Chief Executive Officer
and Chief Financial Officer certifications required by Section 302
of the Sarbanes-Oxley Act. The Company has also submitted the
required annual Chief Executive Officer certification to the New
York Stock Exchange.
References for Page 6
Symplicity HTN-2 Investigators. Renal sympathetic denerva-
tion in patients with treatment-resistant hypertension (The
Symplicity HTN-2 Trial): a randomised controlled trial. Lancet.
2010;376:1903-1909.
Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus
K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT,
Esler M. Catheter-based renal sympathetic denervation for
resistant hypertension: a multicentre safety and proof-of-principle
cohort study. Lancet. 2009;373(9671):1275-1281.
Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal
sympathetic-nerve ablation for uncontrolled hypertension.
N Engl J Med. 2009;361(9):932-934.
For prescribing information for all of the products, visit
medtronic.com.
The following are registered and unregistered trademarks of
Medtronic, Inc. and its affiliated companies:
Activa® PC and RC, Adapta™, AdaptiveStim™, Advisa MRI™ SureScan®, Arctic
Front®, Attain Ability®, CD HORIZON® LEGACY™ (CD HORIZON), Conexus®,
Consulta®, CoreValve®, DBS Therapies™, Endeavor®, Endurant® Abdominal
Stent Graft System, Enlite™, HeartRescueSM, INFUSE® Bone Graft, InterStim®
Therapy, LIFEPAK® 15, LUCAS® Chest Compression System, MAST®,
MasterGraft®, Melody® Transcatheter Pulmonary Valve, MiniMed®
Paradigm® Veo™ System (VEO), MiniMed® Revel™ Systems (Revel), NIM® 3.0
Nerve Monitoring System, O-arm® 3.1.2, O-arm® Imaging Systems,
OptiVol®, Pillar® Palatal Implant System, Progenix®, Protecta™, Resolute™,
Resolute™ Integrity®, Resting Heart® System, RestoreSensor®™, Revo MRI™
SureScan®, Secura®, SmartShock™ Technology, Solera™, Sprint Fidelis™,
StealthStation® S7®, Symplicity® Catheter System, Synergy® Spine 2.0,
Talent® Abdominal Aortic Aneurysm, Vision 3D™.