Medtronic 2011 Annual Report Download - page 66
Download and view the complete annual report
Please find page 66 of the 2011 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
62 Medtronic, Inc.
Notes to Consolidated Financial Statements
(continued)
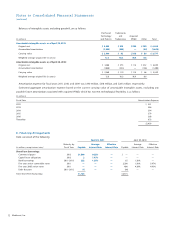
The Company has accounted for the acquisition of Osteotech
as a business combination. The purchase price has been allocated
as follows:
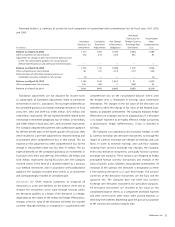
(in millions)
Current assets $ 34
Property, plant, and equipment 21
IPR&D 1
Other intangible assets 46
Goodwill 19
Inventory 41
Other long-term assets 3
Total assets acquire d 165
Current liabilities 19
Other long-term liabilities 15
Long-term deferred tax liabilities, net 8
Total liabilities assumed 42
Net assets acquired $ 123
ATS Medical, Inc. On August 12, 2010, the Company acquired ATS
Medical, Inc. (ATS Medical). ATS Medical is a leading developer,
manufacturer, and marketer of products and services focused on
cardiac surgery, including heart valves and surgical cryoablation
technology. Under the terms of the agreement, ATS Medical
shareholders received $4.00 per share in cash for each share of
ATS Medical common stock that they owned. Total consideration
for the transaction was $394 million, which includes $30 million
of ATS Medical debt and acquired contingent consideration of $10
million. In connection with the acquisition, the Company acquired
$101 million of technology-based intangible assets that had an
estimated useful life of 11 years at the time of acquisition, $6
million of IPR&D, $78 million of net tangible assets, and $209
million of goodwill. The value attributable to IPR&D has been
capitalized as an indefinite-lived intangible asset. The goodwill is
not deductible for tax purposes.
The Company has accounted for the acquisition of ATS Medical
as a business combination. The purchase price has been allocated
as follows:
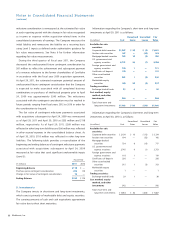
(in millions)
Current assets $ 51
Property, plant, and equipment 7
IPR&D 6
Other intangible assets 101
Goodwill 209
Long-term deferred tax assets, net 34
Total assets acquire d 408
Current liabilities 14
Total liabilities assumed 14
Net assets acquired $ 394
Axon Surgical On June 2, 2010, the Company acquired substantially
all of the assets of Axon Surgical (Axon), a privately-held company.
Prior to the acquisition, the Company distributed a large portion
of Axon’s products. The agreement will allow the Company to
bring to market the next generation of surgeon-directed and
professionally supported spinal neuromonitoring technology and
expand the availability of this technology. Total consideration for
the transaction, net of cash acquired, was $62 million, which
includes the settlement of existing Axon debt. In connection with
the acquisition of Axon, the Company acquired $41 million of
technology-based intangible assets that had an estimated useful
life of 10 years at the time of acquisition, $5 million of tangible
assets, and $16 million of goodwill. The goodwill is deductible for
tax purposes. The Company has accounted for the acquisition of
Axon as a business combination.
Other Acquisitions and Acquisition-Related Items On September 14,
2010, the Company acquired a developer of vascular suturing
products used in connection with cardiovascular and vascular
procedures that require a puncture or incision to the artery. The
terms of the transaction included an up-front payment of $15
million and additional payments of up to $10 million contingent
upon achievement of certain milestones. Total consideration
for the transaction was valued at approximately $21 million,
which includes the estimated fair value of additional milestone-
based contingent consideration of $6 million. The Company has
accounted for this acquisition as a business combination.
During fiscal year 2011, the Company incurred a $15 million
IPR&D charge related to two asset purchases in the CardioVascular
and Surgical Technologies businesses. The Company also incurred
a $15 million IPR&D charge related to a milestone payment
under the existing terms of a royalty-bearing, non-exclusive
patent cross-licensing agreement with NeuroPace, Inc. Product
commercialization related to this technology had not yet been
achieved. As a result, in accordance with authoritative guidance,
the payments for these transactions were immediately expensed
as IPR&D since technological feasibility had not yet been reached
and such technology has no future alternative use. These amounts
are included within acquisition-related items in the consolidated
statement of earnings.
In connection with the Ardian acquisition, the Company
recognized a gain of $85 million on its previously held investment
and incurred approximately $10 million of certain acquisition-
related costs, which include banker fees and other professional
service fees, which were recorded within acquisition-related items
in the consolidated statement of earnings.