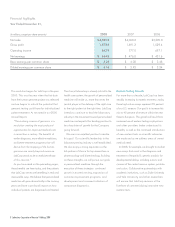
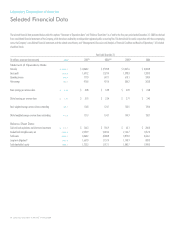
LabCorp 2008 Annual Report Download - page 17
Download and view the complete annual report
Please find page 17 of the 2008 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Two of our companion diagnostic tests
already have reached the marketplace.
The fi rst assay analyzes mutations in the
K-ras gene in patients with metastatic
colon cancer. Analysis of the K-ras gene
for mutation status has been shown to be
a predictive clinical biomarker than can
help stratify patients who are more likely
to respond to treatment with cetuximab
(Erbitux®) or panitumumab (Vectibix®). The
second assay screens for the presence of
the HLA-B*5701 allele in order to identify
patients at higher risk for hypersensitivity
to abacavir, an AIDS treatment drug. Both
tests have experienced increased demand
as prospective studies demonstrated their
clinical utility.
Recently, information has been added to
the drug label for the commonly prescribed
drug warfarin, regarding the value of
patient genotyping to aid in dosing decisions
for this drug. There are indications that
genotyping tests will be a valuable aid in
predicting the appropriate warfarin dose
for specifi c patients.
By determining in advance the specifi c drug
most likely to help a specifi c individual –
with the fewest side effects – we can help
patients receive better, safer and timelier
treatments. Currently, we have relationships
with more than a dozen pharmaceutical and
biotechnology companies for the develop-
ment of companion diagnostics in the fi elds
of oncology and cardiovascular disease.
Our Tandem Labs division works with
pharmaceutical companies to identify
biomarkers that can signal whether a drug
will be compatible with certain individuals.
As pharmaceutical companies develop
new drugs, our clinical trials division works
side-by-side with them to develop companion
diagnostic tests that provide guidance on
the administration of the drug.
Companion
Diagnostics
Mass spectrometry: LabCorp uses state-of-the-art liquid
chromatography tandem mass spectrometry to provide lower
levels of detection, further assisting physicians with their diagnosis
and treatment.
Laboratory Corporation of America® Holdings 2008 15
Beyond The Top Line:
Margin Enhancement
Through Automation
LabCorp is committed to profi table
growth and maintaining our industry-
leading cash fl ow. While personalized
medicine will drive top-line growth,
we have the opportunity to reduce fi xed
costs through automation and capacity
rationalization. Investment in robotics
for pre-analytical processes is an
example of how increased automation
can increase profi tability.
Consider HPV specimen preparation.
Our proprietary system fully automates
the specimen preparation for the
digene Hybrid Capture® 2 assay. This
robotic system uncaps vials, transfers
specimens to custom-designed labware,
dispenses reagents, centrifuges,
incubates and fi nally places prepared
samples in the digene® assay plate. This
system provides uniform processing,
decreases turnaround time and reduces
the labor burden associated with the
HPV assay.