LabCorp 2008 Annual Report Download - page 51
Download and view the complete annual report
Please find page 51 of the 2008 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Notes to Consolidated Financial Statements
(Dollars and shares in millions, except per share data)
Laboratory Corporation of America
Laboratory Corporation of America® Holdings 2008 49
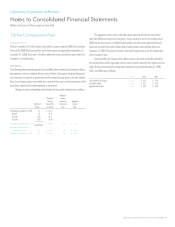
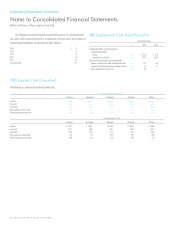
The Company uses the Black-Scholes model to calculate the fair value of the employee’s
purchase right. The fair value of the employee’s purchase right and the assumptions used in its
calculation are as follows:
2008 2007 2006
Fair value of the employee’s purchase right $ 16.10 $ 16.98 $ 11.48
Valuation assumptions
Risk free interest rate 1.2% 4.1% 5.0%
Expected volatility 0.3 0.3 0.1
Expected dividend yield 0.0% 0.0% 0.0%
16) Commitments and Contingent Liabilities
The Company was an appellant in a patent case originally filed by Competitive Technologies, Inc.
and Metabolite Laboratories, Inc. in the United States District Court for the District of Colorado.
After a jury trial, the district court entered judgment against the Company for patent infringement,
with total damages and attorney’s fees payable by the Company of approximately $7.8. The
underlying judgment has been paid. The Company vigorously contested the judgment and appealed
the case ultimately to the United States Supreme Court. On June 22, 2006, the Supreme Court
dismissed the Company’s appeal and the case has been remanded to the District Court for further
proceedings including resolution of a related declaratory judgment action initiated by the Company
addressing the plaintiffs’ claims for post trial damages. On August 15, 2008, the District Court
entered judgment in favor of the Company on all of the plaintiffs’ remaining claims. The plaintiffs
have filed a notice of appeal. The Company does not expect the resolution of these issues to have
a material adverse effect on its financial position, results of operations or liquidity.
The Company is also involved in various claims and legal actions arising in the ordinary
course of business. These matters include, but are not limited to, intellectual property disputes,
professional liability, employee related matters, and inquiries, including subpoenas and other
civil investigative demands, from governmental agencies and Medicare or Medicaid payers and
managed care payers reviewing billing practices or requesting comment on allegations of billing
irregularities that are brought to their attention through billing audits or third parties. The
Company receives civil investigative demands or other inquiries from various governmental
bodies in the ordinary course of its business. Such inquiries can relate to the Company or other
healthcare providers. The Company works cooperatively to respond to appropriate requests for
information. As previously reported on May 22, 2006, the Company received a subpoena from
the California Attorney General seeking documents related to billing to the state’s Medicaid
program. During the third quarter of 2008, the Company received a request for additional
information. The Company continues to cooperate with the California Attorney General’s Office
in responding to the subpoena. In the opinion of management, based upon the advice of counsel
and consideration of all facts available at this time, the ultimate disposition of these matters is
not expected to have a material adverse effect on the financial position, results of operations or
liquidity of the Company.
The Company is also named from time to time in suits brought under the qui tam provisions
of the False Claims Act and comparable state laws. These suits typically allege that the Company
has made false statements and/or certifications in connection with claims for payment from
federal health care programs. They may remain under seal (hence, unknown to the Company)
for some time while the government decides whether to intervene on behalf of the qui tam
plaintiff. Such claims are an inevitable part of doing business in the health care field today
and, in the opinion of management, based upon the advice of counsel and consideration of all
facts available at this time, the ultimate disposition of those qui tam matters presently known
to the Company is not expected to have a material adverse effect on the financial position, results
of operations or liquidity of the Company.
The Company believes that it is in compliance in all material respects with all statutes,
regulations and other requirements applicable to its clinical laboratory operations. The clinical
laboratory testing industry is, however, subject to extensive regulation, and the courts have not
interpreted many of these statutes and regulations. There can be no assurance therefore that
those applicable statutes and regulations might not be interpreted or applied by a prosecutorial,
regulatory or judicial authority in a manner that would adversely affect the Company. Potential
sanctions for violation of these statutes and regulations include significant fines and the loss of
various licenses, certificates and authorizations.
During the fourth quarter of 2008, the Company recorded a $7.5 cumulative revenue
adjustment relating to certain historic overpayments made by Medicare for claims submitted by
a subsidiary of the Company. The Company has initiated communication with the Medicare
carrier to resolve the overpayments.
Under the Company’s present insurance programs, coverage is obtained for catastrophic
exposure as well as those risks required to be insured by law or contract. The Company is
responsible for the uninsured portion of losses related primarily to general, professional and
vehicle liability, certain medical costs and workers’ compensation. The self-insured retentions
are on a per occurrence basis without any aggregate annual limit. Provisions for losses expected
under these programs are recorded based upon the Company’s estimates of the aggregated
liability of claims incurred. At December 31, 2008 and 2007, the Company had provided letters
of credit aggregating approximately $97.4 and $104.8 respectively, primarily in connection with
certain insurance programs and as security for the Company’s contingent obligation to reimburse
up to $200.0 in transition costs under a customer contract. The Company’s availability under
its Revolving Facility is reduced by the amount of these letters of credit.
Effective January 1, 2007, the Company commenced its successful implementation of its
ten-year agreement with UnitedHealthcare Insurance Company (“UnitedHealthcare”) and
became its exclusive national laboratory provider. During the first three years of the ten-year
agreement, the Company has committed to reimburse UnitedHealthcare up to $200.0 for transition
costs related to developing expanded networks in defined markets. Since the inception of this
agreement, approximately $74.6 of such transition payments were billed to the Company by
UnitedHealthcare and approximately $74.4 had been remitted by the Company. Based on