LabCorp 2010 Annual Report Download - page 32
Download and view the complete annual report
Please find page 32 of the 2010 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.30
LABORATORY CORPORATION OF AMERICA
Notes to Consolidated Financial Statements
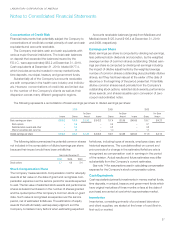
November 19, 2010, the Company sold $925.0 in debt securities,
consisting of $325.0 aggregate principal amount of 3.125%
Senior Notes due May 15, 2016 and $600.0 aggregate principal
amount of 4.625% Senior Notes due November 15, 2020. As
of December 31, 2010 the Company incurred $7.0 of financing
commitment fees, which is included in interest expense for the
year ended December 31, 2010.
The Company incurred approximately $25.7 in professional
fees and expenses in connection with the acquisition of Genzyme
Genetics and other acquisition activity, including significant
costs associated with the Federal Trade Commission’s review
of the Company’s purchase of specified net assets of Westcliff
Medical Laboratories, Inc. These fees and expenses are included
in selling, general and administrative expenses for the year
ended December 31, 2010.
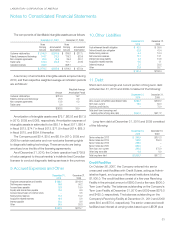
During the year ended December 31, 2010, the Company
also acquired various laboratories and related assets for
approximately $256.1 in cash (net of cash acquired). These
acquisitions were made primarily to extend the Company’s
geographic reach in important market areas and/or enhance
the Company’s scientific differentiation and esoteric testing
capabilities.
During the year ended December 31, 2009, the Company
acquired various other laboratories and related assets for
approximately $212.6 in cash (net of cash acquired). The acqui-
sition activity primarily included the acquisition of Monogram
Biosciences, Inc. (“Monogram”) effective August 3, 2009 for
approximately $160.0 in cash (net of cash acquired). The
Monogram acquisition was made to enhance the Company’s
scientific differentiation and esoteric testing capabilities and
advance the Company’s personalized medicine strategy.
The Monogram purchase consideration has been allocated
to the estimated fair market value of the net assets acquired,
including approximately $63.5 in identifiable intangible assets
(primarily non-tax deductible customer relationships, patents
and technology, and trade name) with weighted-average
useful lives of approximately 15 years; net operating loss tax
assets of approximately $44.8, which are expected to be
realized over a period of 18 years; and residual amount of
non-tax deductible goodwill of approximately $83.6.
Monogram has an active research and development
department, which is primarily focused on the development of
oncology and infectious disease technology. As a result of this
acquisition, the Company incurred approximately $12.1 and
$5.2 of research and development expenses (included in
selling, general and administrative expenses) for the years
ended December 31, 2010 and 2009, respectively.
In connection with the Monogram acquisition, the Company
incurred approximately $2.7 in transaction fees and expenses
(included in selling, general and administrative expenses) for
the year ended December 31, 2009.
During the year ended December 31, 2008, the Company
acquired various laboratories and related assets for approxi-
mately $203.9 in cash (net of cash acquired). These acquisitions
were made primarily to extend the Company’s geographic reach
in important market areas or acquire scientific differentiation
and esoteric testing capabilities.
Effective January 1, 2008 the Company acquired additional
partnership units in its Ontario, Canada (“Ontario”) joint venture
for approximately $140.9 in cash (net of cash acquired), bringing
the Company’s percentage interest owned to 85.6%. Concurrent
with this acquisition, the terms of the joint venture’s partnership
agreement were amended. The amended joint venture’s part-
nership agreement enabled the holders of the noncontrolling
interest to put the remaining partnership units to the Company
in defined future periods, at an initial amount equal to the
consideration paid by the Company in 2008, and subject to
adjustment based on market value formulas contained in the
agreement. The initial difference of $123.0 between the value
of the put and the underlying noncontrolling interest was recorded
as additional noncontrolling interest liability and as a reduction to
additional paid-in capital in the consolidated financial statements.
In December 2009, the Company received notification from
the holders of the noncontrolling interest in the Ontario joint
venture that they intended to put their remaining partnership
units to the Company in accordance with the terms of the joint
venture’s partnership agreement. These units were acquired
on February 8, 2010 for $137.5. On February 17, 2010, the
Company completed a transaction to sell the units acquired from
the previous noncontrolling interest holder to a new Canadian
partner for the same price. As a result of this transaction, the
Company recorded a component of noncontrolling interest in
other liabilities and a component in mezzanine equity. Upon
the completion of these two transactions, the Company’s
financial ownership percentage in the joint venture partnership
remained unchanged at 85.6%. Concurrent with the sale to
the new partner, the partnership agreement for the Ontario
joint venture was amended and restated with substantially