LabCorp 2011 Annual Report Download - page 32
Download and view the complete annual report
Please find page 32 of the 2011 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.30
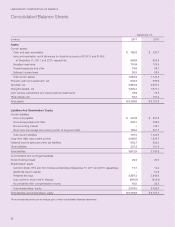
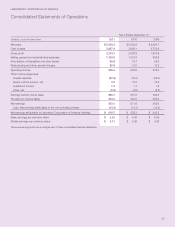
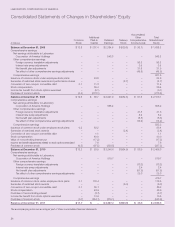
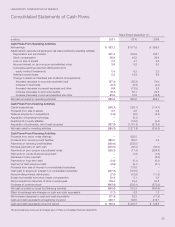
LABORATORY CORPORATION OF AMERICA
Notes to Consolidated Financial Statements
accounting guidance. The guidance is effective for fiscal years
and interim periods beginning after December 15, 2011. The
Company does not believe the adoption of the authoritative
guidance in the first quarter of fiscal 2012 will have an impact
on its consolidated financial position, results of operations or
cash flows.
In May 2011, the FASB issued authoritative guidance to
achieve common fair value measurement and disclosure
requirements between U.S. generally accepted accounting
principles and International Financial Reporting Standards.
This new literature amends current fair value measurement
and disclosure guidance to include increased transparency
around valuation inputs and investment categorization. The
guidance is effective for fiscal years and interim periods
beginning after December 15, 2011. The Company does not
believe the adoption of the authoritative guidance in the first
quarter of fiscal 2012 will have an impact on its consolidated
financial statements.
2. Business Acquisitions
During the twelve months ended December 31, 2011, the
Company acquired various laboratories and related assets
for approximately $137.3 in cash (net of cash acquired).
These acquisitions were made primarily to extend the
Company’s geographic reach in important market areas
and/or enhance the Company’s scientific differentiation and
esoteric testing capabilities.
In April 2011, the Company and Orchid Cellmark Inc.
(“Orchid”) announced that they had entered into a definitive
agreement and plan of merger under which the Company
would acquire all of the outstanding shares of Orchid in a cash
tender offer for $2.80 per share for a total purchase price to
stockholders and optionholders of approximately $85.4. The
tender offer and the merger were subject to customary closing
conditions set forth in the agreement and plan of merger,
including the acquisition in the tender offer of a majority of
Orchid’s fully diluted shares and the expiration or early
termination of the waiting period under the Hart-Scott-Rodino
Antitrust Improvements Act of 1976, as amended (“HSR Act”).
The Company received lawsuits filed by putative classes of
shareholders of Orchid in New Jersey and Delaware state
courts and federal court in New Jersey alleging breaches of
fiduciary duty and/or other violations of state law arising out
of the proposed acquisition of Orchid. Both Orchid and the
Company are named in the lawsuits. The federal court lawsuit
was subsequently dismissed and the New Jersey state court
actions have been stayed. The remaining Delaware lawsuits
have been consolidated and will be vigorously defended.
On December 8, 2011, the Company announced that it
had reached an agreement with the U.S. Federal Trade
Commission allowing the Company to complete its acquisition
of Orchid. Under the terms of the proposed consent decree
that was accepted by the FTC for public comment, the
Company is required to divest certain assets of Orchid’s U.S.
government paternity business following closing of the
acquisition. On December 16, 2011, the Company sold those
assets to DNA Diagnostics Center, a privately held provider of
DNA paternity testing. The Company completed its acquisition
of Orchid on December 15, 2011. It has recorded a $2.8 non-
deductible loss on the divestiture of Orchid’s U.S. government
paternity business in Other Income and Expense in the
accompanying Consolidated Statements of Operations.
The Orchid purchase consideration has been allocated to
the estimated fair market value of the net assets acquired,
including approximately $28.8 in identifiable intangible assets
(primarily non-tax deductible customer relationships, trade
names and trademarks) with weighted-average useful lives of
approximately 12 years; $9.1 in deferred tax liabilities (relating
to identifiable intangible assets); net operating loss tax assets
of approximately $20.2, which are expected to be realized
over a period of 20 years; and a residual amount of non-tax
deductible goodwill of approximately $27.2. The purchase
price allocation for this acquisition is preliminary and subject
to adjustment based on changes in the fair value of working
capital and other assets and liabilities on the effective
acquisition date and final valuation of intangible assets.
The partnership units of the holders of the noncontrolling
interest in the Ontario, Canada (“Ontario”) joint venture were
acquired by the Company on February 8, 2010 for $137.5. On
February 17, 2010, the Company completed a transaction to
sell the units acquired from the previous noncontrolling interest
holder to a new Canadian partner for the same price. As a
result of this transaction, the Company recorded a component
of noncontrolling interest in other liabilities and a component in
mezzanine equity as the joint venture’s partnership agreement
enabled one of the holders of the noncontrolling interest to put
its remaining partnership units to the Company in defined
future periods, at an initial amount equal to the consideration