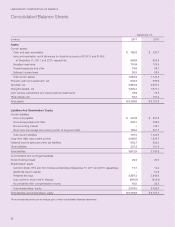
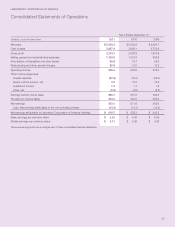
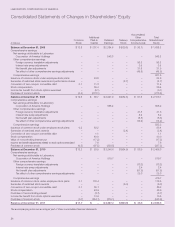
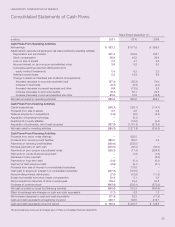
LabCorp 2011 Annual Report Download - page 33
Download and view the complete annual report
Please find page 33 of the 2011 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.31
LABORATORY CORPORATION OF AMERICA
Notes to Consolidated Financial Statements
paid by that holder in 2010, and subject to adjustment based
on market value formulas contained in the agreement. Upon
the completion of these two transactions, the Company’s
financial ownership percentage in the joint venture partnership
remained unchanged at 85.6%. Concurrent with the sale to
the new partner, the partnership agreement for the Ontario
joint venture was amended and restated with substantially
the same terms as the previous agreement.
On October 14, 2011, the Company issued notice to a
noncontrolling interest holder in the Ontario joint venture of its
intent to purchase the holder’s partnership units in accordance
with the terms of the joint venture’s partnership agreement.
On November 28, 2011, this purchase was completed for a
total purchase price of $147.9 (CN$151.7) as outlined in the
partnership agreement (CN$147.8 plus certain adjustments
relating to cash distribution hold backs made to finance recent
business acquisitions and capital expenditures). The purchase
of these additional partnership units brings the Company’s
percentage interest owned to 98.2%.
Net sales of the Ontario joint venture were $309.4
(CN$306.0), $280.0 (CN$288.5) and $247.5 (CN$281.3) for
the twelve months ended December 31, 2011, 2010 and
2009, respectively.
On December 1, 2010, the Company acquired Genzyme
Genetics, a business unit of Genzyme Corporation, for
approximately $925.2 in cash (net of cash acquired). The
Genzyme Genetics acquisition was made to expand the
Company’s capabilities in reproductive, genetic, hematology-
oncology and clinical trials central laboratory testing, enhance
the Company’s esoteric testing capabilities and advance the
Company’s personalized medicine strategy.
The Genzyme Genetics purchase consideration has been
allocated to the estimated fair market value of the net assets
acquired, including approximately $279.6 in identifiable
intangible assets (primarily customer relationships and trade
name) with weighted-average useful lives of approximately
23 years; and residual amount of goodwill of approximately
$537.8. Approximately $810.5 of the total intangible value will
be amortizable for tax purposes over 15 years.
On October 28, 2010, in conjunction with the acquisition
of Genzyme Genetics, the Company entered into a $925.0
bridge term loan credit agreement. The Company replaced
and terminated the bridge term loan credit agreement in
November 2010 by making an offering in the debt capital
markets. On November 19, 2010, the Company sold $925.0
in debt securities, consisting of $325.0 aggregate principal
amount of 3.125% Senior Notes due May 15, 2016 and $600.0
aggregate principal amount of 4.625% Senior Notes due
November 15, 2020. As of December 31, 2010 the Company
incurred $7.0 of financing commitment fees, which was included
in interest expense for the year ended December 31, 2010.
The Company incurred approximately $25.7 in professional
fees and expenses in connection with the acquisition of
Genzyme Genetics and other acquisition activity, including
significant costs associated with the Federal Trade Commis-
sion’s review of the Company’s purchase of specified net
assets of Westcliff Medical Laboratories, Inc. These fees and
expenses are included in selling, general and administrative
expenses for the year ended December 31, 2010.
During the year ended December 31, 2010, the
Company also acquired various laboratories and related
assets for approximately $256.1 in cash (net of cash
acquired). These acquisitions were made primarily to extend
the Company’s geographic reach in important market areas
and/or enhance the Company’s scientific differentiation and
esoteric testing capabilities.
During the year ended December 31, 2009, the Company
acquired various laboratories and related assets for approxi-
mately $212.6 in cash (net of cash acquired). The acquisition
activity primarily included the acquisition of Monogram
Biosciences, Inc. (“Monogram”) effective August 3, 2009 for
approximately $160.0 in cash (net of cash acquired). The
Monogram acquisition was made to enhance the Company’s
scientific differentiation and esoteric testing capabilities and
advance the Company’s personalized medicine strategy.
The Monogram purchase consideration has been allocated
to the estimated fair market value of the net assets acquired,
including approximately $63.5 in identifiable intangible assets
(primarily non-tax deductible customer relationships, patents
and technology, and trade name) with weighted-average
useful lives of approximately 15 years; net operating loss tax
assets of approximately $44.8, which are expected to be
realized over a period of 18 years; and residual amount of
non-tax deductible goodwill of approximately $83.6.
Monogram has an active research and development
department, which is primarily focused on the development of
oncology and infectious disease technology. As a result of this
acquisition, the Company incurred approximately $8.5, $12.1