LabCorp 2012 Annual Report Download
Download and view the complete annual report
Please find the complete 2012 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
2012 Annual Report
Table of contents
-
Page 1
2012 Annual Report -
Page 2
..., hospitals, clinical labs, and pharmaceutical companies. To learn more about our company, visit our Web site at: www.labcorp.com. LabCorp A Premier Health Care Services Company Attractive Market Strong Financial Fundamentals Clear Mission Superior Execution Five-Pillar Strategy Revenue and... -
Page 3
... developing and implementing a suite of data-driven knowledge services that optimize decision-making, improve health outcomes and reduce treatment costs. Our ï¬rst strategic pillar is to deploy capital to investments that enhance our business and return capital to shareholders. The 2012 acquisition... -
Page 4
... lab results, make lab appointments, pay bills, set up automatic alerts and notiï¬cations and manage health information for the entire family. Our open platform approach to Electronic Medical Record ("EMR") connectivity beneï¬ts customers by allowing them to connect seamlessly to LabCorp directly... -
Page 5
... management of cardiovascular disorders. This program, available through our Beacon® platform and our customer's EMR, uses innovative result displays, analytics and trending as well as Cardiovascular Disease Risk Assessment decision support for individualized lipid assessment and patient counseling... -
Page 6
... acquiring physician practices. Our capabilities provide an end-to-end lab solution for these customers, meeting the requirements of new care models with population health management tools, decision support programs, patient counseling, integrated clinical reports and patientcentric data solutions... -
Page 7
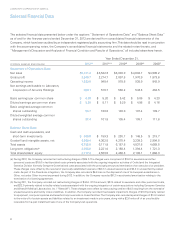
฀ 2012 Financial Summary Table of Contents Selected Financial Data Management's Discussion and Analysis of Financial Condition and Results of Operations Report of Management on Internal Control Over Financial Reporting Report of Independent Registered Public Accounting Firm Consolidated Balance ... -
Page 8
... of certain acquisitions including Genzyme Genetics and Westcliff Medical Laboratories, Inc. ("Westcliff"). These charges were offset by restructuring credits of $4.8 resulting from the reversal of unused severance and facility closure liabilities. In addition, the Company recorded fixed assets... -
Page 9
...addition, the Company recorded a special charge of $6.2 related to the write-off of development costs incurred on systems abandoned during the year. The Company incurred approximately $25.7 in professional fees and expenses in connection with the acquisition of Genzyme Genetics and other acquisition... -
Page 10
... Company's testing volume is dependent on patient visits to physician offices and other providers of health care. Volume of testing generally declines during the year-end holiday periods and other major holidays. In addition, volume declines due to inclement weather may reduce net revenues and cash... -
Page 11
... collection trends resulting from process improvement programs within the Company's billing department and field operations. These decreases in selling, general and administrative expenses were partially offset by $9.9 in fees from the MEDTOX acquisition recorded during 2012. Selling, general... -
Page 12
...of employee severance benefits as a result of changes in cost estimates on the restructuring initiatives. In addition, the Company recorded a special charge of $6.2 related to the write-off of development costs incurred on systems abandoned during the year. From time to time, the Company implements... -
Page 13
... a % of income before tax 2012 2011 2010 $ 359.4 $ 333.0 $ 344.0 38.1% 38.4% 37.6% Liquidity, Capital Resources and Financial Position The Company's strong cash-generating capability and financial condition typically have provided ready access to capital markets. The Company's principal source of... -
Page 14
LABORATORY CORPORATION OF AMERICA Management's Discussion and Analysis of Financial Condition and Results of Operations (in millions) can achieve this through use of its Revolving Credit Facility and its ready access to debt capital markets. In the event that the Company needs additional liquidity,... -
Page 15
... 15. On December 1, 2010, the acquisition of Genzyme Genetics was funded by the proceeds from the issuance of these Notes ($915.4) and with cash on hand. During 2012, the Company purchased $516.5 of its stock representing 5.9 shares. As of December 31, 2012, the Company had remaining outstanding... -
Page 16
... business day of the calendar quarter, which is 5:00 p.m., New York City time, on Friday, March 29, 2013. If notices of conversion are received, the Company plans to settle the cash portion of the conversion obligation with cash on hand and/or borrowings under the revolving credit facility. Credit... -
Page 17
... the partnership agreement (CN$147 .8 plus certain adjustments relating to cash distribution hold backs made to finance recent business acquisitions and capital expenditures). The purchase of these additional partnership units brings the Company's percentage interest owned to 98.2%. The contractual... -
Page 18
...client list price, less any negotiated discount. Patient sales are recorded at the Company's patient fee schedule, net of any discounts negotiated with physicians on behalf of their patients. The Company bills third-party payers in two ways - fee-for-service and capitated agreements. Fee-for-service... -
Page 19
...plans have been closed to new participants. Employees participating in the Company Plan and the PEP no longer earn service-based credits, but continue to earn interest credits. In addition, effective January 1, 2010, all employees eligible for the defined contribution retirement plan (the "401K Plan... -
Page 20
... in the normal course of business, generally related to the testing and reporting of laboratory test results. The Company maintains excess insurance which limits the Company's maximum exposure on individual claims. The Company estimates a liability that represents the ultimate exposure for aggregate... -
Page 21
... public filings, press releases and discussions with Company management, including: 1. changes in federal, state, local and third-party payer regulations or policies or other future reforms in the health care system (or in the interpretation of current regulations), new insurance or payment systems... -
Page 22
... to develop, perform, or market the Company's tests or operate its business; 26. failure in the Company's information technology systems resulting in an increase in testing turnaround time or billing processes or the failure to meet future regulatory or customer information technology, data security... -
Page 23
...Company addresses its exposure to market risks, principally the market risk associated with changes in interest rates, through a controlled program of risk management that includes, from time to time, the use of derivative financial instruments such as interest rate swap agreements. Although, as set... -
Page 24
...of December 31, 2012, the Company maintained effective internal control over financial reporting. Management reviewed the results of its assessment with the Audit Committee of the Company's Board of Directors. PricewaterhouseCoopers LLP , an independent registered public accounting firm, who audited... -
Page 25
... financial position of Laboratory Corporation of America Holdings and its subsidiaries at December 31, 2012 and 2011, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2012 in conformity with accounting principles generally accepted... -
Page 26
LABORATORY CORPORATION OF AMERICA Consolidated Balance Management's Discussion Sheets and Analysis of Financial Condition and Results of Operations (in millions) December 31, (In Millions) 2012 2011 Assets Current assets: Cash and cash equivalents Accounts receivable, net of allowance for ... -
Page 27
LABORATORY CORPORATION OF AMERICA Consolidated Statements Management's Discussion of and Operations Analysis of Financial Condition and Results of Operations (in millions) Years Ended December 31, (In Millions, Except Per Share Data) 2012 $ 5,671.4 3,421.7 2,249.7 1,114.6 86.3 25.3 1,023.5 (94.5) ... -
Page 28
... 2009 Net earnings attributable to Laboratory Corporation of America Holdings Other comprehensive earnings, net of tax Issuance of common stock under employee stock plans Surrender of restricted stock and performance share awards Conversion of zero-coupon convertible debt Stock compensation Value of... -
Page 29
...other Net cash provided by operating activities Cash Flows From Investing Activities: Capital expenditures Proceeds from sale of assets Deferred payments on acquisitions Acquisition of licensing technology Investments in equity affiliates Acquisition of businesses, net of cash acquired Net cash used... -
Page 30
... sales Routine Testing Genomic and Esoteric Testing Ontario, Canada Total 2012 $ 3,246.6 2,089.8 335.0 $ 5,671.4 2011 $ 3,143.9 2,089.0 309.4 $ 5,542.3 2010 $ 2,995.4 1,728.5 280.0 $ 5,003.9 Use of Estimates The preparation of financial statements in conformity with generally accepted accounting... -
Page 31
... cash on deposit in an escrow account for an acquisition in Canada that closed in January 2013. Substantially all of the Company's accounts receivable are with companies in the health care industry and individuals. However, concentrations of credit risk are limited due to the number of the Company... -
Page 32
... starting from the time that the preliminary project stage is completed and the Company commits to funding a project until the project is substantially complete and the software is ready for its intended use. Capitalized costs include direct material and service costs and payroll and payroll-related... -
Page 33
.... Professional Liability The Company is self-insured (up to certain limits) for professional liability claims arising in the normal course of business, generally related to the testing and reporting of laboratory test results. The Company estimates a liability that represents the ultimate exposure... -
Page 34
... Business Acquisitions On July 31, 2012, the Company completed its acquisition of MEDTOX Scientific, Inc. ("MEDTOX"), a provider of high quality specialized laboratory testing services and on-site/ point-of-collection testing (POCT) devices, for $236.4 in cash, excluding transaction fees. The MEDTOX... -
Page 35
... a business unit of Genzyme Corporation, for approximately $925.2 in cash (net of cash acquired). The Genzyme Genetics acquisition was made to expand the Company's capabilities in reproductive, genetic, hematologyoncology and clinical trials central laboratory testing, enhance the Company's esoteric... -
Page 36
LABORATORY CORPORATION OF AMERICA Notes to Consolidated Financial Statements The Genzyme Genetics purchase consideration has been allocated to the estimated fair market value of the net assets acquired, including approximately $279.6 in identifiable intangible assets (primarily customer ... -
Page 37
... on all major business decisions as well as providing other participating rights to each partner. The equity method investments represent the Company's purchase of shares in clinical diagnostic companies. The investments are accounted for under the equity method of accounting as the Company does not... -
Page 38
...conduct diagnostic testing services in Canada. This indefinite lived asset is tested for impairment annually as described in Note 1. 9. Accrued Expenses and Other Employee compensation and benefits Self-insurance reserves Accrued taxes payable Royalty and license fees payable Restructuring reserves... -
Page 39
LABORATORY CORPORATION OF AMERICA Notes to Consolidated Financial Statements 10. Other Liabilities Post-retirement benefit obligation Defined benefit plan obligation Restructuring reserves Self-insurance reserves Acquisition related reserves Deferred revenue Other December 31, 2012 $ 60.7 122.5 19... -
Page 40
... the last business day of the calendar quarter, which is 5:00 p.m., New York City time, on Friday, March 29, 2013. If notices of conversion are received, the Company plans to settle the cash portion of the conversion obligation with cash on hand and/or borrowings under the revolving credit facility... -
Page 41
... of Genzyme Genetics was funded by the net proceeds from the issuance of these Notes ($915.4) and with cash on hand. The Senior Notes due January 31, 2013 bear interest at the rate of 5.5% per annum from February 1, 2003, payable semi-annually on February 1 and August 1. The Senior Notes due 2015... -
Page 42
... of its self-insurance reserves and employee compensation and benefits, as described in Note 9. The valuation allowance increased from $14.4 in 2011 to $18.4 in 2012. The increase in the valuation allowance is primarily due to a current year foreign capital loss resulting from the disposition... -
Page 43
... grant under the Plan. This Plan was approved by shareholders at the 2012 annual meeting. Stock Options The following table summarizes grants of non-qualified options made by the Company to officers, key employees, and non-employee directors under all plans. Stock options are generally granted at an... -
Page 44
... For 2012, 2011 and 2010, expense related to the Company's stock option plan totaled $21.5, $24.9 and $20.7 , respectively. Restricted Stock and Performance Shares The Company grants restricted stock and performance shares ("non-vested shares") to officers, key employees, and non-employee directors... -
Page 45
... from time to time in suits brought under the qui tam provisions of the False Claims Act and comparable state laws. These suits typically allege that the Company has made false statements and/or certifications in connection with claims for payment from federal or state health care programs. They... -
Page 46
...the United States Department of Health & Human Services Office of Inspector General's regional office in New York. On August 17 , 2011, the Southern District of New York unsealed a False Claims Act lawsuit, United States of America ex rel. NPT Associates v. Laboratory Corporation of America Holdings... -
Page 47
... State of California, County of San Francisco. The lawsuit alleges that the defendants committed unlawful and unfair business practices, and violated various other state laws by changing screening codes to diagnostic codes on laboratory test orders, thereby resulting in customers being responsible... -
Page 48
... damages, as well as recovery of attorney's fees, and legal expenses. The Company will vigorously defend the lawsuit. On June 7 , 2012, the Company was served with a putative class action lawsuit, Ann Baker Pepe v. Genzyme Corporation and Laboratory Corporation of America Holdings, filed in the... -
Page 49
... Plan of $11.3, $0.0 and $0.0 in 2012, 2011 and 2010, respectively. The PEP covers the Company's senior management group. Prior to 2010, the PEP provided for the payment of the difference, if any, between the amount of any maximum limitation on annual benefit payments under the Employee Retirement... -
Page 50
...a limited number of existing employees of the subsidiary. This plan is unfunded and the Company's policy is to fund benefits as claims are incurred. The effect on operations of the post-retirement medical plan is shown in the following table: Year ended December 31, Service cost for benefits earned... -
Page 51
... of $0.4. The following assumed benefit payments under the Company's post-retirement benefit plan, which reflect expected future service, as appropriate, and were used in the calculation of projected benefit obligations, are expected to be paid as follows: 2013 2014 2015 2016 2017 Years 2018-2022... -
Page 52
...- 2011 $ 2.4 19. Supplemental Cash Flow Information Years Ended December 31, 2012 Supplemental schedule of cash flow information: Cash paid during period for: Interest $ 77.5 Income taxes, net of refunds 306.2 Disclosure of non-cash financing and investing activities: Surrender of restricted stock... -
Page 53
..., Investor Relations 336-436-5076 Center for Molecular Biology and Pathology 800-345-4363 Center for Occupational Testing 800-833-3984 Center for Esoteric Testing 800-334-5161 Paternity/Identity 800-762-4344 LabCorp Drug Development Laboratory Services 877-788-8861 Web Site www.labcorp.com Transfer... -
Page 54
Laboratory Corporation of America® Holdings 358 South Main Street Burlington, NC 27215 336-584-5171 www.labcorp.com