LabCorp 2012 Annual Report Download - page 34
Download and view the complete annual report
Please find page 34 of the 2012 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.32
LABORATORY CORPORATION OF AMERICA
Notes to Consolidated Financial Statements
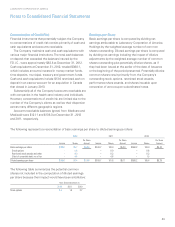
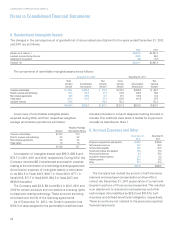
Fair Value of Financial Instruments
Fair value measurements for financial assets and liabilities
are determined based on the assumptions that a market
participant would use in pricing an asset or liability. A three-
tiered fair value hierarchy draws distinctions between market
participant assumptions based on (i) observable inputs such
as quoted prices in active markets (Level 1), (ii) inputs other
than quoted prices in active markets that are observable
either directly or indirectly (Level 2) and (iii) unobservable
inputs that require the Company to use present value and
other valuation techniques in the determination of fair value
(Level 3).
Research and Development
The Company expenses research and development costs
as incurred.
New Accounting Pronouncements
In June 2011, the FASB issued authoritative guidance on
the presentation of comprehensive income. Specifically,
this literature requires an entity to present components of
net earnings and other comprehensive income in one
continuous statement, referred to as the statement of
comprehensive income, or in two separate, but consecutive
statements. The authoritative guidance eliminates the
option to report other comprehensive income and its
components in the statement of changes in shareholders’
equity. While the authoritative guidance changes the
presentation of comprehensive income, there are no
changes to the components that are recognized in net
earnings or other comprehensive income under current
accounting guidance. The Company adopted this guidance
during the first quarter of 2012 and elected to present
comprehensive income in two separate, but consecutive
statements and has applied the new presentation to the
prior period presented. The adoption of this authoritative
guidance in the first quarter of fiscal 2012 did not have an
impact on the Company’s consolidated financial position,
results of operations or cash flows.
In February 2013, the FASB issued an amendment
to existing guidance regarding the reporting of amounts
reclassified out of accumulated other comprehensive
income. The amendment requires an entity to present
information about reclassification adjustments from
accumulated other comprehensive income in its annual
financial statements in a single note or on the face of
the financial statements. The amendment is effective
prospectively for reporting periods beginning after
December 15, 2012. We do not expect this amendment to
have a significant impact on the Company’s Consolidated
Financial Statements.
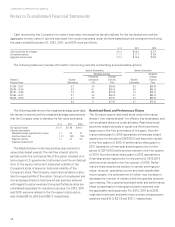
2. Business Acquisitions
On July 31, 2012, the Company completed its acquisition
of MEDTOX Scientific, Inc. (“MEDTOX”), a provider of high
quality specialized laboratory testing services and on-site/
point-of-collection testing (POCT) devices, for $236.4 in
cash, excluding transaction fees. The MEDTOX acquisition
was made to extend the Company’s specialty toxicology
testing group and enhance the Company’s scientific
differentiation and esoteric testing capabilities.
The MEDTOX purchase consideration has been
allocated to the estimated fair market value of the net assets
acquired, including approximately $78.0 in identifiable
intangible assets (primarily non-tax deductible customer
relationships, trade names and trademarks) with weighted-
average useful lives of approximately 18 years; $33.2 in
deferred tax liabilities (relating to identifiable intangible
assets); and a residual amount of non-tax deductible
goodwill of approximately $154.2.
During the year ended December 31, 2012, the Company
also acquired various other laboratories and related assets
for approximately $95.8 in cash (net of cash acquired). These
acquisitions were made primarily to extend the Company’s
geographic reach in important market areas and/or enhance
the Company’s scientific differentiation and esoteric testing
capabilities. The purchase price allocations for certain of
these acquisitions are preliminary and subject to adjustment
based on changes in the fair value of working capital and
other assets and liabilities on the effective acquisition dates
and final valuation of intangible assets.
In April 2011, the Company and Orchid Cellmark Inc.
(“Orchid”) announced that they had entered into a definitive
agreement and plan of merger under which the Company
would acquire all of the outstanding shares of Orchid in a
cash tender offer for $2.80 per share for a total purchase
price to stockholders and optionholders of approximately
$85.4. The tender offer and the merger were subject to
customary closing conditions set forth in the agreement