LabCorp 2012 Annual Report Download - page 45
Download and view the complete annual report
Please find page 45 of the 2012 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
43
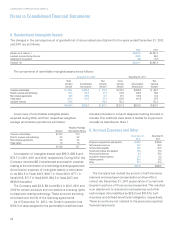
The following table shows a summary of non-vested
shares for the year ended December 31, 2012:
Weighted-
Average
Number of Grant Date
Shares Fair Value
Non-vested at January 1, 2012 0.6 $ 74.39
Granted 0.2 91.62
Adjustment 0.1 61.19
Vested (0.3) 64.60
Non-vested at December 31, 2012 0.6 $ 84.91
As of December 31, 2012, there was $17.2 of total
unrecognized compensation cost related to non-vested
restricted stock and performance share-based compensation
arrangements granted under the stock incentive plans. That
cost is expected to be recognized over a weighted-average
period of 1.6 years.
Employee Stock Purchase Plan
The Company has an employee stock purchase plan, begun
in 1997 and amended in 1999, 2004, 2008 and 2012, with
6.3 shares of common stock authorized for issuance. The
plan permits substantially all employees to purchase a limited
number of shares of Company stock at 85% of market value.
The Company issues shares to participating employees
semi-annually in January and July of each year. Approximately
0.2 shares were purchased by eligible employees in 2012,
2011 and 2010, respectively. For 2012, 2011 and 2010, expense
related to the Company’s employee stock purchase plan
was $4.9, $2.7 and $2.0, respectively.
The Company uses the Black-Scholes model to calculate
the fair value of the employee’s purchase right. The fair
value of the employee’s purchase right and the assumptions
used in its calculation are as follows:
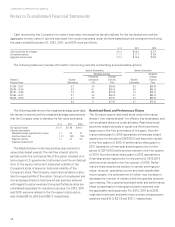
2012 2011 2010
Fair value of the employee’s purchase right $ 23.02 $ 15.58 $ 15.39
Valuation assumptions
Risk free interest rate 0.1% 0.1% 0.2%
Expected volatility 0.2 0.2 0.2
Expected dividend yield – – –
15. Commitments and Contingent Liabilities
The Company is involved from time to time in various
claims and legal actions, including arbitrations, class actions,
and other litigation (including those described in more detail
below), arising in the ordinary course of business. Some of
these actions involve claims that are substantial in amount.
These matters include, but are not limited to, intellectual
property disputes, professional liability, employee related
matters, and inquiries, including subpoenas and other civil
investigative demands, from governmental agencies and
Medicare or Medicaid payers and managed care payers
reviewing billing practices or requesting comment on allega-
tions of billing irregularities that are brought to their attention
through billing audits or third parties. The Company receives
civil investigative demands or other inquiries from various
governmental bodies in the ordinary course of its business.
Such inquiries can relate to the Company or other healthcare
providers. The Company works cooperatively to respond to
appropriate requests for information.
The Company is also named from time to time in suits
brought under the qui tam provisions of the False Claims
Act and comparable state laws. These suits typically allege
that the Company has made false statements and/or certifi-
cations in connection with claims for payment from federal
or state health care programs. They may remain under seal
(hence, unknown to the Company) for some time while the
government decides whether to intervene on behalf of the
qui tam plaintiff. Such claims are an inevitable part of doing
business in the health care field today.
The Company believes that it is in compliance in all
material respects with all statutes, regulations and other
requirements applicable to its clinical laboratory operations.
The clinical laboratory testing industry is, however, subject
to extensive regulation, and the courts have not interpreted
many of these statutes and regulations. There can be no
assurance therefore that those applicable statutes and
regulations will not be interpreted or applied by a prose cu-
torial, regulatory or judicial authority in a manner that would
adversely affect the Company. Potential sanctions for violation
of these statutes and regulations include significant fines and
the loss of various licenses, certificates and authorizations.
LABORATORY CORPORATION OF AMERICA
Notes to Consolidated Financial Statements