LabCorp 2012 Annual Report Download - page 36
Download and view the complete annual report
Please find page 36 of the 2012 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.34
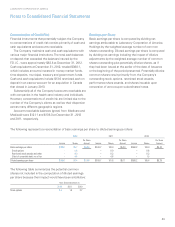
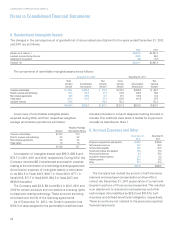
The Genzyme Genetics purchase consideration has been
allocated to the estimated fair market value of the net assets
acquired, including approximately $279.6 in identifiable
intangible assets (primarily customer relationships and trade
name) with weighted-average useful lives of approximately
23 years; and residual amount of goodwill of approximately
$537.8. Approximately $810.5 of the total intangible value
will be amortizable for tax purposes over 15 years.
On October 28, 2010, in conjunction with the acquisition
of Genzyme Genetics, the Company entered into a
$925.0 bridge term loan credit agreement. The Company
replaced and terminated the bridge term loan credit
agreement in November 2010 by making an offering in the
debt capital markets. On November 19, 2010, the Company
sold $925.0 in debt securities, consisting of $325.0
aggregate principal amount of 3.125% Senior Notes due
May 15, 2016 and $600.0 aggregate principal amount of
4.625% Senior Notes due November 15, 2020. As of
December 31, 2010 the Company incurred $7.0 of financing
commitment fees, which were included in interest expense
for the year ended December 31, 2010.
The Company incurred approximately $25.7 in professional
fees and expenses in connection with the acquisition of
Genzyme Genetics and other acquisition activity, including
significant costs associated with the Federal Trade
Commission’s review of the Company’s purchase of
specified net assets of Westcliff Medical Laboratories,
Inc. These fees and expenses are included in selling,
general and administrative expenses for the year ended
December 31, 2010.
During the year ended December 31, 2010, the Company
also acquired various laboratories and related assets for
approximately $256.1 in cash (net of cash acquired). These
acquisitions were made primarily to extend the Company’s
geographic reach in important market areas and/or enhance
the Company’s scientific differentiation and esoteric
testing capabilities.
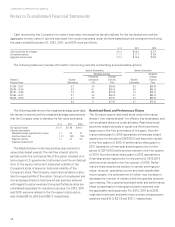
3. Restructuring and Other Special Charges
During 2012, the Company recorded net restructuring
charges of $25.3. The charges were comprised of $16.2 in
severance and other personnel costs and $19.6 in facility-
related costs primarily associated with the ongoing
integration activities of Orchid and the Integrated Genetics
Division (formerly Genzyme Genetics) and costs associated
with the previously announced termination of an executive
vice president. These charges were offset by the reversal of
previously established reserves of $6.3 in unused severance
and $4.2 in unused facility-related costs.
As part of the Clearstone integration, the Company also
recorded a $6.9 loss on the disposal of one of its European
subsidiaries in Other, net under Other income (expenses)
during 2012.
During 2011, the Company recorded net restructuring
charges of $44.6. Of this amount, $27.4 related to severance
and other personnel costs, and $22.0 primarily related to
facility-related costs associated with the ongoing integration
of certain acquisitions including Genzyme Genetics and
Westcliff. These charges were offset by restructuring credits
of $4.8, resulting from the reversal of unused severance and
facility closure liabilities. In addition, the Company recorded
fixed assets impairment charges of $18.9 primarily related
to equipment, computer systems and leasehold improve-
ments in closed facilities. The Company also recorded special
charges of $14.8 related to the write-off of certain assets
and liabilities related to an investment made in prior years,
along with a $2.6 write-off of an uncollectible receivable
from a past installment sale of one of the Company’s
lab operations.
During 2010, the Company recorded net restructuring
charges of $5.8 primarily related to the closing of redundant
and underutilized facilities. Of this amount, $8.0 related to
severance and other employee costs for employees primarily
in the affected facilities, and $3.1 related to contractual
obligations associated with leased facilities and other facility
related costs. The Company also reduced its prior restructuring
accruals by $5.3, comprised of $4.7 of previously recorded
facility costs and $0.6 of employee severance benefits as a
result of changes in cost estimates on the restructuring initia-
tives. In addition, the Company recorded a special charge of
$6.2 related to the write-off of development costs incurred
on systems abandoned during the year.
LABORATORY CORPORATION OF AMERICA
Notes to Consolidated Financial Statements