Pfizer 2012 Annual Report Download - page 29
Download and view the complete annual report
Please find page 29 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
28
2012 Financial Report
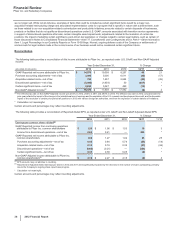
NEW DRUG CANDIDATES IN LATE-STAGE DEVELOPMENT
CANDIDATE INDICATION
ALO-02 A Mu-type opioid receptor agonist for the management of moderate-to-severe pain when a continuous,
around-the-clock opioid analgesic is needed for an extended period of time
Dacomitinib A pan-HER tyrosine kinase inhibitor for the treatment of previously treated advanced non-small cell lung
cancer
Inotuzumab ozogamicin An antibody drug conjugate, consisting of an anti-CD22 monotherapy antibody linked to a cytotoxic
agent, calicheamycin, for the treatment of aggressive Non-Hodgkin's Lymphoma and acute
lymphoblastic leukemia
MnB rLP2086
(PF-05212366)
A prophylactic vaccine for prevention of Neisseria meningitidis serogroup B invasive disease in
adolescents and young adults (ages 11 - 25)
Palbociclib (PD-0332991) An oral and selective reversible inhibitor of the CDK 4 and 6 kinases for the treatment of patients with
ER positive, HER2 negative advanced breast cancer
Tanezumab(a) An anti-nerve growth factor monoclonal antibody for the treatment of pain (on clinical hold)
(a) Following requests by the FDA in 2010, we suspended and subsequently terminated worldwide the osteoarthritis, chronic low back pain and painful diabetic
peripheral neuropathy studies of tanezumab. The FDA's requests followed a small number of reports of osteoarthritis patients treated with tanezumab who
experienced the worsening of osteoarthritis leading to total joint replacement and also reflected the FDA's concerns regarding the potential for such events in
other patient populations. In December 2010, the FDA placed a clinical hold on all other anti-nerve growth factor therapies under clinical investigation in the U.S.
Studies of tanezumab in cancer pain were allowed to continue. Extensive analyses were undertaken of all total joint replacements reported in studies of
tanezumab. The results of these analyses and the conclusions drawn were provided to the FDA. On March 12, 2012, the FDA's Arthritis Advisory Committee
met to discuss the future development of nerve growth factor inhibitors, including tanezumab. The Committee voted that there is a role for the ongoing
development of nerve growth factor inhibitors in conditions such as osteoarthritis and for the management of pain associated with conditions other than
osteoarthritis for which there are no agents with demonstrated analgesic effect. We submitted a Clinical Hold Complete Response to the FDA on July 31, 2012.
On August 28, 2012, the FDA removed the clinical hold completely from the tanezumab program for all indications. On December 14, 2012, the FDA placed a
new partial clinical hold on the development of nerve growth factor inhibitors, including tanezumab. The partial clinical hold was based on peripheral nervous
system effects observed in animal studies conducted with nerve growth factor inhibitors by other companies. Current and future studies of tanezumab in cancer
pain are not affected by this partial clinical hold. We intend to work with the FDA to determine the appropriate path forward.
Additional product-related programs are in various stages of discovery and development. Also, see the discussion in the “Our Business
Development Initiatives” section of this Financial Review.
COSTS AND EXPENSES
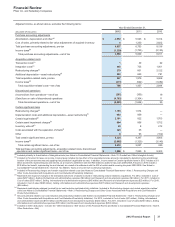
Cost of Sales
Year Ended December 31, % Change
(MILLIONS OF DOLLARS) 2012 2011 2010 12/11 11/10
Cost of sales $11,334 $14,076 $14,788 (19)(5)
2012 v. 2011
Cost of sales decreased 19% in 2012, compared to 2011, primarily due to:
• lower purchase accounting charges, primarily reflecting the fair value adjustments to acquired inventory from Wyeth and King that was
subsequently sold;
• lower costs related to our cost-reduction and productivity initiatives and acquisition-related costs, as well as the benefits generated from
the ongoing productivity initiatives to streamline the manufacturing network;
• reduced manufacturing volumes related to products that lost exclusivity in various markets; and
• the favorable impact of foreign exchange of 3%,
partially offset by:
• an unfavorable shift in geographic, product and business mix due to products that lost exclusivity in various markets.
2011 v. 2010
Cost of sales decreased 5% in 2011, compared to 2010, primarily due to:
• lower purchase accounting charges, primarily reflecting the fair value adjustments to acquired inventory from Wyeth that was
subsequently sold; and
• savings associated with our cost-reduction and productivity initiatives,
partially offset by:
• the addition of costs from legacy King’s operations;
• the Puerto Rico excise tax (for additional information, see the “Provision for Taxes on Income” section of this Financial Review);