Pfizer 2012 Annual Report Download - page 67
Download and view the complete annual report
Please find page 67 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
66
2012 Financial Report
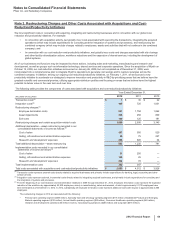
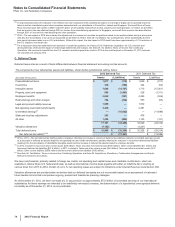
The unaudited pro forma consolidated results do not purport to project the future results of operations of the combined company nor do they
reflect the expected realization of any cost savings associated with the acquisition. The unaudited pro forma consolidated results reflect the
historical financial information of Pfizer and King, adjusted for the following pre-tax amounts:
• Elimination of King's historical intangible asset amortization expense (approximately $6 million in 2011 and $116 million in 2010).
• Additional amortization expense (approximately $15 million in 2011 and $190 million in 2010) related to the fair value of identifiable
intangible assets acquired.
• Additional depreciation expense (approximately $3 million in 2011 and $35 million in 2010) related to the fair value adjustment to
property, plant and equipment acquired.
• Adjustment related to the fair value adjustments to acquisition-date inventory estimated to have been sold (elimination of $160 million
charge in 2011 and addition of $160 million charge in 2010).
• Adjustment for acquisition-related costs directly attributable to the acquisition (elimination of $224 million of charges in 2011 and addition
of $224 million of charges in 2010, reflecting charges incurred by both King and Pfizer).
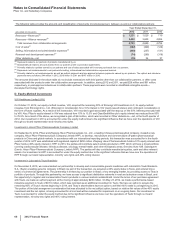
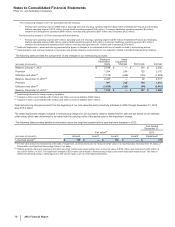
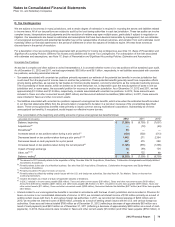
FoldRx Pharmaceuticals, Inc.
On October 6, 2010, we completed our acquisition of FoldRx Pharmaceuticals, Inc. (FoldRx), a privately held drug discovery and clinical
development company. FoldRx's lead product candidate, Vyndaqel (tafamidis meglumine), is a first-in-class oral therapy for the treatment of
transthyretin familial amyloid polyneuropathy (TTR-FAP). The total consideration for the acquisition was approximately $400 million, which
consisted of an upfront payment to FoldRx's shareholders of approximately $200 million and contingent consideration with an estimated
acquisition-date fair value of approximately $200 million. The contingent consideration consists of up to $455 million in additional payments
that are contingent upon the attainment of certain regulatory and revenue milestones. Payments under the contingent consideration
arrangement were $225 million in 2012, as a regulatory milestone was achieved. In connection with this Specialty Care acquisition, we
recorded approximately $500 million in Identifiable intangible assets––In-process research and development, approximately $160 million in net
deferred tax liabilities and approximately $60 million in Goodwill. In 2012, we recorded a decrease in the fair value of the contingent
consideration of approximately $42 million and in 2011, we recorded an increase in the fair value of the contingent consideration of
approximately $85 million.
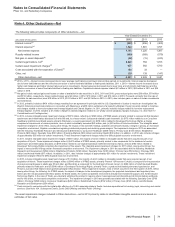
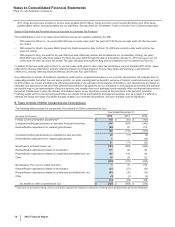
B. Divestitures
Nutrition Business
On November 30, 2012, we completed the sale of our Nutrition business to Nestlé for $11.85 billion in cash, and recognized a gain of
approximately $4.8 billion, net of tax, in Gain/(loss) on sale of discontinued operations––net of tax. The divested business includes:
• our former Nutrition operating segment and certain prenatal vitamins previously commercialized by the Pfizer Consumer Healthcare
operating segment; and
• other associated amounts, such as direct manufacturing costs, enabling support functions and other costs not charged to the business,
purchase-accounting impacts, acquisition-related costs, impairment charges, restructuring charges and implementation costs
associated with our cost reduction/productivity initiatives, all of which are reported outside our operating segment results.
The operating results of this business are reported as Income/(loss) from discontinued operations––net of tax in the consolidated statements
of income for all periods presented. In addition, in the consolidated balance sheet as of December 31, 2011, the assets and liabilities
associated with this discontinued operation are classified as Assets of discontinued operations and other assets held for sale and Liabilities of
discontinued operations, as appropriate.
While the full purchase price of $11.85 billion was received on November 30, the sale of the business was not completed in certain non-U.S.
jurisdictions where regulatory review of the transaction remains ongoing. In these jurisdictions, which represent a relatively small portion of the
Nutrition business, we continue to operate the business on an interim basis pending regulatory approval or divestiture to a third party buyer.
These interim arrangements, pursuant to which Pfizer operates the business for the net economic benefit of Nestlé and is indemnified by
Nestlé against any risk associated with such operations during the interim period, are expected to conclude by the end of 2013 and the sale of
these certain jurisdictions are expected to be completed by the end of 2013. As such, and as we have already received all of the expected
proceeds from the sale, and as Nestlé is contractually obligated to complete the transaction (or permit us to divest the delayed businesses to a
third party buyer on its behalf) regardless of the outcome of any pending regulatory reviews, we have treated these delayed-close businesses
as sold for accounting purposes.
In connection with the sale transaction, we also entered into certain transitional agreements designed to ensure and facilitate the orderly
transfer of business operations to the buyer. These agreements primarily relate to administrative services, which are generally to be provided
for a period of 2 to 18 months. We will also manufacture and supply certain prenatal vitamin products for a transitional period. These
agreements are not material and none confers upon us the ability to influence the operating and/or financial policies of the Nutrition business
subsequent to the sale.