Pfizer 2012 Annual Report Download - page 61
Download and view the complete annual report
Please find page 61 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
60
2012 Financial Report
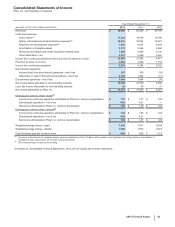
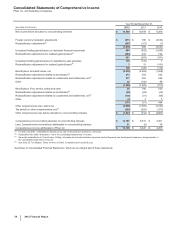
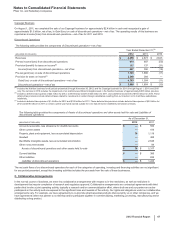
G. Revenues
Revenue Recognition—We record revenues from product sales when the goods are shipped and title passes to the customer. At the time of
sale, we also record estimates for a variety of sales deductions, such as sales rebates, discounts and incentives, and product returns. When
we cannot reasonably estimate the amount of future product returns and/or other sales deductions, we record revenues when the risk of
product return and/or additional sales deductions has been substantially eliminated. We record sales of certain of our vaccines to the U.S.
government as part of the Pediatric Vaccine Stockpile program; these rules require that for fixed commitments made by the U.S. government,
we record revenues when risk of ownership for the completed product has been passed to the U.S. government. There are no specific
performance obligations associated with products sold under this program.
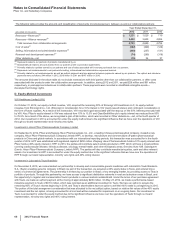
Deductions from Revenues––As is typical in the biopharmaceutical industry, our gross product sales are subject to a variety of deductions that
generally are estimated and recorded in the same period that the revenues are recognized and primarily represent rebates and discounts to
government agencies, wholesalers, distributors and managed care organizations with respect to our biopharmaceutical products. These
deductions represent estimates of the related obligations.
Specifically:
• In the U.S., we record provisions for pharmaceutical Medicaid, Medicare and performance-based contract rebates based upon our
experience ratio of rebates paid and actual prescriptions written during prior quarters. We apply the experience ratio to the respective
period’s sales to determine the rebate accrual and related expense. This experience ratio is evaluated regularly to ensure that the
historical trends are as current as practicable. In addition, to account for the impacts of the Patient Protection and Affordable Care Act, as
amended by the Health Care and Education Reconciliation Act (together, U.S. Healthcare Legislation), we also consider the increase in
minimum rebate and extension of Medicaid prescription drug rebates for drugs dispensed to enrollees. We estimate discounts on
branded prescription drug sales to Medicare Part D participants in the Medicare “coverage gap,” also known as the “doughnut hole,”
based on historical experience of beneficiary prescriptions and consideration of the utilization that is expected to result from the discount
in the coverage gap. We evaluate this estimate regularly to ensure that the historical trends and future expectations are as current as
practicable. For performance-based contract rebates, we also consider current contract terms, such as changes in formulary status and
discount rates.
• Outside the U.S., the majority of our pharmaceutical rebates, discounts and price reductions (collectively, sales allowances) are
contractual or legislatively mandated and our estimates are based on actual invoiced sales within each period; both of these elements
help to reduce the risk of variations in the estimation process. Some European countries base their rebates on the government’s
unbudgeted pharmaceutical spending, and we use an estimated allocation factor (based on historical payments) and total revenues by
country against our actual invoiced sales to project the expected level of reimbursement. We obtain third-party information that helps us
to monitor the adequacy of these accruals.
• Provisions for pharmaceutical chargebacks (primarily reimbursements to wholesalers for honoring contracted prices to third parties)
closely approximate actual as we settle these deductions generally within two to five weeks of incurring the liability.
• Provisions for pharmaceutical returns are based on a calculation for each market that incorporates the following, as appropriate: local
returns policies and practices; returns as a percentage of sales; an understanding of the reasons for past returns; estimated shelf life by
product; an estimate of the amount of time between shipment and return or lag time; and any other factors that could impact the estimate
of future returns, such as loss of exclusivity, product recalls or a changing competitive environment. Generally, returned products are
destroyed, and customers are refunded the sales price in the form of a credit.
• We record sales incentives as a reduction of revenues at the time the related revenues are recorded or when the incentive is offered,
whichever is later. We estimate the cost of our sales incentives based on our historical experience with similar incentives programs.
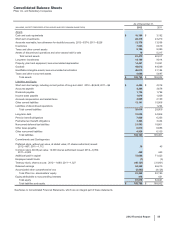
Our accruals for Medicaid rebates, Medicare rebates, performance-based contract rebates, sales allowances and chargebacks were $3.8
billion as of December 31, 2012, and $4.8 billion as of December 31, 2011, and substantially all are included in Other current liabilities.
Amounts recorded for sales deductions can result from a complex series of judgments about future events and uncertainties and can rely
heavily on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Note 1C. Basis of
Presentation and Significant Accounting Policies: Estimates and Assumptions.
Taxes collected from customers relating to product sales and remitted to governmental authorities are presented on a net basis; that is, they
are excluded from Revenues.
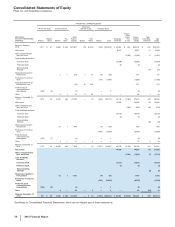
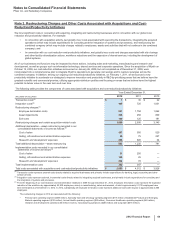
Collaborative Arrangements—Payments to and from our collaboration partners are presented in our consolidated statements of income based
on the nature of the arrangement (including its contractual terms), the nature of the payments and applicable accounting guidance. Under co-
promotion agreements, we record the amounts received from our partners as alliance revenues, a component of Revenues, when our co-
promotion partners are the principal in the transaction and we receive a share of their net sales or profits. Alliance revenues are recorded
when our co-promotion partners ship the product and title passes to their customers. The related expenses for selling and marketing these
products are included in Selling, informational and administrative expenses. In collaborative arrangements where we manufacture a product
for our partner, we record revenues when our partner sells the product and title passes to its customer. All royalty payments to collaboration
partners are included in Cost of sales.