LabCorp 2013 Annual Report Download - page 16
Download and view the complete annual report
Please find page 16 of the 2013 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.12
LABORATORY CORPORATION OF AMERICA
Management’s Discussion and Analysis
of Financial Condition and Results of Operations (in millions)
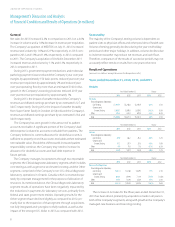
The Company has discussed its intention to increase its ratio of
total debt to consolidated Earnings Before Interest, Taxes, Depreciation,
and Amortization (“EBITDA”) over time from 2.0 to 1.0 as of
December 2012 to 2.5 to 1.0. The Company believes that it can
achieve this through the use of its Revolving Credit Facility and its
ready access to debt capital markets. As of December 31, 2013,
the ratio of total debt to consolidated EBITDA was 2.4 to 1.0. The
Company continues to monitor the debt capital markets and,
given current market conditions, believes it can readily increase its
ratio of total debt to consolidated EBITDA. The Company believes
that its cash from operations, in combination with cash on hand
and borrowing capacity, will be sufficient to satisfy its obligations
in 2014 and beyond.
Operating Activities
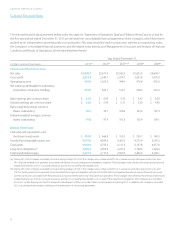
In 2013, the Company’s operations provided $818.7 of cash,
reflecting the Company’s solid business results. The decrease in
cash provided from operations in 2013 as compared with 2012
is primarily attributable to the delays and denials of coverage
for existing tests by some payers after implementation of newly
established molecular pathology codes as well as government
payment reductions. These delays, denials and government
reductions reduced year over year operating cash flow by more
than an estimated $100.0. The Company continues to focus on
efforts to increase cash collections from all payers and to generate
ongoing improvements to the claim submission processes.
In 2012, the Company’s operations provided $841.4 of cash,
reflecting the Company’s solid business results. The Company
continued to focus on efforts to increase cash collections from
all payers and to generate ongoing improvements to the claim
submission processes.
Investing Activities
Capital expenditures were $202.2, $173.8 and $145.7 for 2013, 2012
and 2011, respectively. The increase in capital spending in 2013
was related to certain integration and cost savings initiatives started
by the Company. The Company expects capital expenditures
of approximately $185.0 to $205.0 in 2014. Such expenditures are
expected to be funded by cash flow from operations, as well
as borrowings under the Company’s Revolving Credit Facility
as needed.
The Company remains committed to growing its business
through strategic acquisitions and licensing agreements. The
Company has invested a total of $632.9 over the past three years
in strategic business acquisitions, including MEDTOX Scientific in
2012 and Orchid in 2011. These acquisitions have helped strengthen
the Company’s geographic presence along with expanding
capabilities in the specialty testing operations. The Company
believes the acquisition market remains attractive with a number of
opportunities to strengthen its scientific capabilities, grow esoteric
testing capabilities and increase presence in key geographic areas.
The Company has invested a total of $2.9 over the past three years
in licensing new testing technologies and had $41.0 net book value
of capitalized patents, licenses and technology as of December 31,
2013. While the Company continues to believe its strategy of
entering into licensing and technology distribution agreements
with the developers of leading-edge technologies will provide
future growth in revenues, there are certain risks associated with
these investments. These risks include, but are not limited to, the
failure of the licensed technology to gain broad acceptance in
the marketplace and/or that insurance companies, managed care
organizations, or Medicare and Medicaid will not approve
reimbursement for these tests at a level commensurate with
the costs of running the tests. Any or all of these circumstances
could result in impairment in the value of the related capitalized
licensing costs.
Financing Activities
On December 21, 2011, the Company entered into a Credit
Agreement (the “Credit Agreement”) providing for the Revolving
Credit Facility, a five-year $1,000.0 senior unsecured revolving credit
facility with Bank of America, N.A., acting as Administrative Agent,
Barclays Capital as Syndication Agent, and a group of financial
institutions as lending parties. As part of the new Revolving Credit
Facility, the Company repaid all of the outstanding principal balances
of $318.8 on its existing term loan facility and $235.0 on its existing
revolving credit facility. In conjunction with the repayment and
cancellation of its old credit facility, the Company recorded
approximately $1.0 of remaining unamortized debt costs as
interest expense in the accompanying Consolidated Statements
of Operations for the year ended December 31, 2011.
There were no balances outstanding on the Company’s
Revolving Credit Facility at December 31, 2013 or December 31,
2012. The Revolving Credit Facility bears interest at varying rates
based upon a base rate or LIBOR plus (in each case) a percentage
based on the Company’s debt rating with Standard & Poor’s and
Moody’s Rating Services.