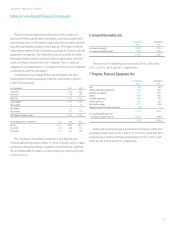
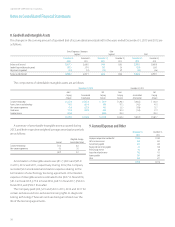
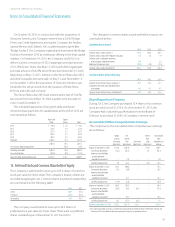
LabCorp 2013 Annual Report Download - page 49
Download and view the complete annual report
Please find page 49 of the 2013 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.45
investigative demand from the State of Texas Office of the Attorney
General requesting documents related to its billing to Texas
Medicaid. The Company is cooperating with these requests.
On May 2, 2013, the Company was served with a False Claims
Act lawsuit, State of Georgia ex rel. Hunter Laboratories, LLC and Chris
Riedel v. Quest Diagnostics Incorporated, et al., filed in the State Court
of Fulton County, Georgia. The lawsuit, filed by a competitor labora-
tory, alleges that the Company overcharged Georgia’s Medicaid
program. The case has been removed to the United States District
Court for the Northern District of Georgia. The lawsuit seeks actual
and treble damages and civil penalties for each alleged false claim,
as well as recovery of costs, attorney’s fees, and legal expenses.
The government filed a notice declining to intervene in the case.
The Company will vigorously defend the lawsuit.
On August 19, 2013, the Company was served with a False
Claims Act lawsuit, Commonwealth of Virginia ex rel. Hunter
Laboratories, LLC and Chris Riedel v. Quest Diagnostics Incorporated, et
al., filed in the Circuit Court of Fairfax County, Virginia. The lawsuit,
filed by a competitor laboratory, alleges that the Company over-
charged Virginia’s Medicaid program. The case has been removed
to the United States District Court for the Eastern District of Virginia.
The lawsuit seeks actual and treble damages and civil penalties for
each alleged false claim, as well as recovery of costs, attorney’s fees,
and legal expenses. The government filed a notice declining to
intervene in the case. The Company’s Motion to Dismiss was
granted and the plaintiffs have been granted the right to replead
their complaint. The Company will vigorously defend the lawsuit.
In October 2011, a putative stockholder of the Company made
a letter demand through his counsel for inspection of documents
related to policies and procedures concerning the Company’s
Board of Directors’ oversight and monitoring of the Company’s
billing and claim submission process. The letter also seeks docu-
ments prepared for or by the Board regarding allegations from the
California ex rel. Hunter Laboratories, LLC et al. v. Quest Diagnostics
Incorporated, et al., lawsuit and documents reviewed and relied
upon by the Board in connection with the settlement of that
lawsuit. The Company is responding to the request pursuant to
Delaware law.
On November 18, 2011, the Company received a letter from U.S.
Senators Baucus and Grassley requesting information regarding the
Company’s relationships with its largest managed care customers.
The letter requests information about the Company’s contracts and
financial data regarding its managed care customers. Company
representatives met with Senate Finance Committee staff after
receiving the request and subsequently produced documents in
response. The Company continues to cooperate with the request
for information.
On February 27, 2012, the Company was served with a False
Claims Act lawsuit, United States ex rel. Margaret Brown v. Laboratory
Corporation of America Holdings and Tri-State Clinical Laboratory
Services, LLC, filed in the United States District Court for the Southern
District of Ohio, Western Division. The lawsuit alleges that the
defendants submitted false claims for payment for laboratory testing
services performed as a result of financial relationships that violated
the federal Stark and anti-kickback laws. The Company owned 50%
of Tri-State Clinical Laboratory Services, LLC, which was dissolved in
June of 2011. The lawsuit seeks actual and treble damages and civil
penalties for each alleged false claim, as well as recovery of costs,
attorney’s fees, and legal expenses. The U.S. government has not
intervened in the lawsuit. The Company will vigorously defend
the lawsuit.
On June 7, 2012, the Company was served with a putative class
action lawsuit, Yvonne Jansky v. Laboratory Corporation of America,
et al., filed in the Superior Court of the State of California, County of
San Francisco. The lawsuit alleges that the defendants committed
unlawful and unfair business practices, and violated various other
state laws by changing screening codes to diagnostic codes on
laboratory test orders, thereby resulting in customers being respon-
sible for co-payments and other debts. The lawsuit seeks injunctive
relief, actual and punitive damages, as well as recovery of attorney’s
fees, and legal expenses. The Company will vigorously defend
the lawsuit.
On June 7, 2012, the Company was served with a putative class
action lawsuit, Ann Baker Pepe v. Genzyme Corporation and Laboratory
Corporation of America Holdings, filed in the United States District
Court for the District of Massachusetts. The lawsuit alleges that the
defendants failed to preserve DNA samples allegedly entrusted to
the defendants and thereby breached a written agreement with
plaintiff and violated state laws. The lawsuit seeks injunctive relief,
actual, double and treble damages, as well as recovery of attorney’s
fees and legal expenses. The Company will vigorously defend
the lawsuit.
LABORATORY CORPORATION OF AMERICA
Notes to Consolidated Financial Statements