Pfizer 2012 Annual Report Download - page 22
Download and view the complete annual report
Please find page 22 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
2012 Financial Report
21
Chantix/Champix An aid to smoking cessation
treatment
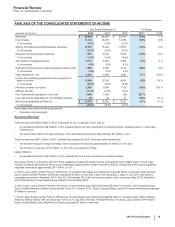
670 720 755 (7) (5)
Pristiq Depression 630 577 466 924
ReFacto AF/Xyntha Hemophilia 584 506 404 15 25
Zoloft Depression and certain anxiety
disorders
541 573 532 (6) 8
Revatio Pulmonary arterial hypertension
(PAH)
534 535 481 —11
Medrol Inflammation 523 510 455 312
Zosyn/Tazocin Antibiotic 484 636 952 (24)(33)
Zithromax/Zmax Bacterial infections 435 453 415 (4) 9
Effexor Depression and certain anxiety
disorders
425 678 1,718 (37)(61)
Prevnar/Prevenar (7-valent) Vaccine for prevention of
pneumococcal disease
399 488 1,253 (18)(61)
Fragmin Anticoagulant 381 382 341 —12
Relpax Treat the symptoms of migraine
headache
368 341 323 86
Rapamune Immunosuppressant 346 372 388 (7) (4)
Cardura Hypertension/Benign prostatic
hyperplasia
338 380 413 (11)(8)
Tygacil Antibiotic 335 298 324 12 (8)
Aricept(a) Alzheimer's disease 326 450 454 (28)(1)
Xanax XR Anxiety disorders 274 306 307 (10)—
BMP2 Development of bone and cartilage 263 340 400 (23)(15)
Sulperazon Antibiotic 262 218 213 20 2
Diflucan Fungal infections 259 265 278 (2) (5)
Caduet Reduction of LDL cholesterol and
hypertension
258 538 527 (52)2
Neurontin Seizures 235 289 322 (19)(10)
Dalacin/Cleocin Antibiotic for bacterial infections 232 192 214 21 (10)
Unasyn Injectable antibacterial 228 231 244 (1) (5)
Metaxalone/Skelaxin(b) Muscle relaxant 223 203 —10 *
Inspra High blood pressure 214 195 157 10 24
Toviaz Overactive bladder 207 187 137 11 36
Somavert Acromegaly 197 183 157 817
Alliance revenues(c) Various 3,492 3,630 4,084 (4) (11)
All other(d) Various 8,289 8,584 8,118 (3) 6
(a) Represents direct sales under license agreement with Eisai Co., Ltd.
(b) Legacy King product. King’s operations are included in our financial statements commencing from the acquisition date of January 31, 2011. Therefore, our
results for 2010 do not include King’s results of operations.
(c) Enbrel (in the U.S. and Canada), Spiriva, Rebif, Aricept and Exforge.
(d) Includes sales of generic atorvastatin.
* Calculation not meaningful.
Certain amounts and percentages may reflect rounding adjustments.
Biopharmaceutical—Selected Product Descriptions
• Lyrica is indicated for the management of post-herpetic neuralgia, neuropathic pain associated with diabetic peripheral neuropathy, the
management of fibromyalgia, neuropathic pain due to spinal cord injury, and as adjunctive therapy for adult patients with partial onset
seizures in the U.S. For certain countries outside the U.S., Lyrica is indicated for neuropathic pain (peripheral and central), the
management of fibromyalgia, adjunctive treatment of epilepsy and general anxiety disorder. Lyrica recorded increases in worldwide
revenues of 13% in 2012, compared to 2011. There was strong operational performance in international markets in 2012, including
Japan, where Lyrica was launched in 2010 as the first product approved for the peripheral neuropathic pain (NeP) indication.
Internationally, Lyrica revenues increased 14% in 2012, compared to 2011, with the growth due to a focus on enhancing the neuropathic
pain diagnosis and treatment rates, the successful re-launch of the general anxiety disorder indication in the EU and physician education
regarding neuropathic pain in Japan. Foreign exchange had an unfavorable impact on international revenues of 5% in 2012, compared to
2011. In the U.S., revenues increased 10% in 2012, compared to 2011. Notwithstanding these increases, U.S. revenues continue to be
affected by increased competition from generic versions of competitive medicines, as well as managed care pricing and formulary
pressures.