Pfizer 2012 Annual Report Download - page 4
Download and view the complete annual report
Please find page 4 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
2012 Financial Report
3
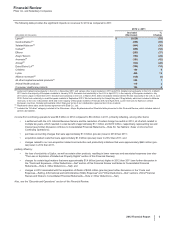
The following table provides the significant impacts on revenues for 2012 as compared to 2011:
2012 v. 2011
(MILLIONS OF DOLLARS)
Increase/
(Decrease)
%
Change
Lipitor(a) $ (5,629) (59)
Geodon/Zeldox(a) (669)(65)
Xalatan/Xalacom(a) (444)(36)
Caduet(a) (280)(52)
Effexor (253)(37)
Zosyn/Tazocin (152)(24)
Aromasin(a) (151)(42)
Aricept(b) (124)(28)
Detrol/Detrol LA(a) (122)(14)
Celebrex 196 8
Lyrica 465 13
Alliance revenues(a) (138)(4)
All other biopharmaceutical products(c) 525 7
Animal Health products 115 3
Consumer Healthcare products 184 6
(a) Lipitor and Caduet lost exclusivity in the U.S. in November 2011 and various other major markets in 2011 and 2012. Xalatan lost exclusivity in the U.S. in March
2011 and in the majority of European markets in January 2012. Aromasin lost exclusivity in the U.S. in April 2011, in the majority of European markets in July
2011 and in Japan in November 2011. Geodon lost exclusivity in the U.S. in March 2012. Detrol immediate release (Detrol IR) lost exclusivity in the U.S. in June
2012. Detrol lost exclusivity in most European markets in September 2012. We lost exclusivity for Aricept 5mg and 10mg tablets, which are included in Alliance
revenues, in the U.S. in November 2010 and in the majority of European markets in February 2012 and April 2012. Lower revenues for Spiriva in certain
European countries, Canada and Australia reflect final-year terms of our collaboration agreements in those markets.
(b) Represents direct sales under license agreement with Eisai Co., Ltd.
(c) Includes the “All other” category included in the Revenues—Major Biopharmaceutical Products table presented in this Financial Review, which includes sales of
generic atorvastatin.
Income from continuing operations was $9.5 billion in 2012 compared to $8.4 billion in 2011, primarily reflecting, among other items:
• a settlement with the U.S. Internal Revenue Service and the resolution of certain foreign tax audits in 2012, all of which related to
multiple tax years, which resulted in a tax benefit of approximately $1.1 billion and $310 million, respectively, representing tax and
interest (see further discussion in Notes to Consolidated Financial Statements––Note 5A. Tax Matters: Taxes on Income from
Continuing Operations);
• purchase accounting charges that were approximately $1.8 billion (pre-tax) lower in 2012 than 2011;
• acquisition-related costs that were approximately $1.0 billion (pre-tax) lower in 2012 than 2011; and
• charges related to our non-acquisition related cost-reduction and productivity initiatives that were approximately $645 million (pre-
tax) lower in 2012 than 2011,
partially offset by:
• the loss of exclusivity of Lipitor, as well as certain other products, resulting in lower revenues and associated expenses (see also
"The Loss or Expiration of Intellectual Property Rights" section of this Financial Review);
• charges for certain legal matters that were approximately $1.4 billion (pre-tax) higher in 2012 than 2011 (see further discussion in
the “Costs and Expenses––Other Deductions––Net” section of this Financial Review and Notes to Consolidated Financial
Statements––Note 4. Other Deductions––Net); and
• charges in 2012 associated with the separation of Zoetis of $325 million (pre-tax) (see further discussion in the “Costs and
Expenses––Selling, Informational and Administrative (SI&A) Expenses" and "Other Deductions––Net” sections of this Financial
Review and Notes to Consolidated Financial Statements––Note 4. Other Deductions––Net).
Also, see the “Discontinued Operations” section of this Financial Review.