Pfizer 2012 Annual Report Download - page 26
Download and view the complete annual report
Please find page 26 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
2012 Financial Report
25
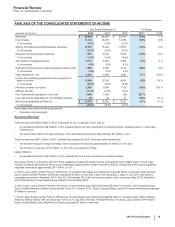
The following table provides additional information by operating segment about our research and development expenses (see also Notes to
Consolidated Financial Statements––Note 18. Segment, Geographic and Other Revenue Information):
Research and Development Expenses
Year Ended December 31, % Change
(MILLIONS OF DOLLARS) 2012 2011 2010 12/11 11/10
Primary Care(a) $1,009 $1,307 $1,473 (23)(11)
Specialty Care and Oncology(a) 1,401 1,561 1,624 (10)(4)
Established Products and Emerging Markets(a) 403 441 452 (9) (2)
Other(a), (b) 693 425 428 63 (1)
Worldwide Research and Development/Pfizer Medical(c) 2,835 3,337 3,709 (15)(10)
Corporate and Other(d) 1,529 2,003 1,797 (24)11
Total Research and Development Expenses $7,870 $9,074 $9,483 (13)(4)
(a) Our operating segments, in addition to their sales and marketing responsibilities, are responsible for certain development activities. Generally, these
responsibilities relate to additional indications for in-line products and IPR&D projects that have achieved proof-of-concept. R&D spending may include upfront
and milestone payments for intellectual property rights.
(b) Includes the Animal Health operating segment and the Consumer Healthcare operating segment. The increase in 2012 primarily relates to a $250 million
payment to AstraZeneca to obtain the exclusive global over-the-counter rights to Nexium.
(c) Worldwide Research and Development is generally responsible for human health research projects until proof-of-concept is achieved, and then for transitioning
those projects to the appropriate business unit for possible clinical and commercial development. R&D spending may include upfront and milestone payments
for intellectual property rights. This organization also has responsibility for certain science-based and other platform-services organizations, which provide
technical expertise and other services to the various R&D projects. Worldwide Research and Development is also responsible for all human-health-related
regulatory submissions and interactions with regulatory agencies, including all safety event activities. Pfizer Medical is responsible for external affairs relating to
all therapeutic areas, providing Pfizer-related medical information to healthcare providers, patients and other parties, and quality assurance and regulatory
compliance activities, which include conducting clinical trial audits and readiness reviews. The decreases in 2012 compared to 2011 and in 2011 compared to
2010 result from cost savings associated with the R&D productivity initiative announced on February 1, 2011 (see the “Restructuring Charges and Other Costs
Associated with Acquisitions and Cost-Reduction/Productivity Initiatives” section of this Financial Review).
(d) Corporate and other includes unallocated costs, primarily facility costs, information technology, share-based compensation, and restructuring related costs. The
decrease in 2012 primarily results from cost savings associated with the R&D productivity initiative announced on February 1, 2011 and to a lesser extent from
lower charges relating to implementing our cost-reduction and productivity initiatives (see the “Restructuring Charges and Other Costs Associated with
Acquisitions and Cost-Reduction/Productivity Initiatives” section of this Financial Review).
Our human health R&D spending is conducted through a number of matrix organizations––Research Units, within our Worldwide Research
and Development organization, are generally responsible for research assets (assets that have not yet achieved proof-of-concept); Business
Units are generally responsible for development assets (assets that have achieved proof-of-concept); and science-based and other platform-
services organizations.
We take a holistic approach to our human health R&D operations and manage the operations on a total-company basis through our matrix
organizations described above. Specifically, a single committee, co-chaired by members of our R&D and commercial organizations, is
accountable for aligning resources among all of our human health R&D projects and for ensuring that our company is focusing its R&D
resources in the areas where we believe that we can be most successful and maximize our return on investment. We believe that this
approach also serves to maximize accountability and flexibility.
Our Research Units are organized in a variety of ways (by therapeutic area or combinations of therapeutic areas, by discipline, by location,
etc.) to enhance flexibility, cohesiveness and focus. Because of our structure, we can rapidly redeploy resources, within a Research Unit,
between various projects as necessary because the workforce shares similar skills, expertise and/or focus.
Our platform-services organizations, where a significant portion of our R&D spending occurs, provide technical expertise and other services to
the various R&D projects, and are organized into science-based functions such as Pharmaceutical Sciences, Chemistry, Drug Safety, and
Development Operations, and non-science-based functions, such as Facilities, Business Technology and Finance. As a result, within each of
these functions, we are able to migrate resources among projects, candidates and/or targets in any therapeutic area and in most phases of
development, allowing us to react quickly in response to evolving needs.
Generally, we do not disaggregate total R&D expense by development phase or by therapeutic area since, as described above, we do not
manage a significant portion of our R&D operations by development phase or by therapeutic area. Further, as we are able to adjust a
significant portion of our spending quickly, as conditions change, also as described above, we believe that any prior-period information about
R&D expense by development phase or by therapeutic area would not necessarily be representative of future spending.
Product Developments—Biopharmaceutical
We continue to invest in R&D to provide potential future sources of revenues through the development of new products, as well as through
additional uses for in-line and alliance products. Notwithstanding our efforts, there are no assurances as to when, or if, we will receive
regulatory approval for additional indications for existing products or any of our other products in development.
We continue to closely evaluate our global research and development function and pursue strategies intended to improve innovation and
overall productivity in R&D by prioritizing areas that we believe have the greatest scientific and commercial promise, utilizing appropriate risk/
return profiles and focusing on areas that we believe have the highest potential to deliver value in the near term and over time. To that end, our