Pfizer 2012 Annual Report Download - page 47
Download and view the complete annual report
Please find page 47 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
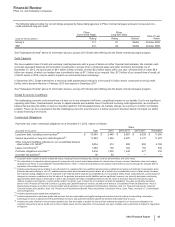
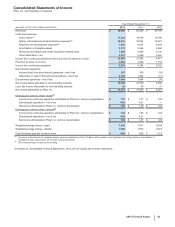
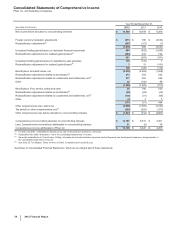
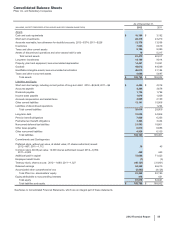
Financial Review
Pfizer Inc. and Subsidiary Companies
46
2012 Financial Report
• any significant breakdown, infiltration or interruption of our information technology systems and infrastructure;
• legal defense costs, insurance expenses, settlement costs, the risk of an adverse decision or settlement and the
adequacy of reserves related to product liability, patent protection, government investigations, consumer, commercial,
securities, antitrust, environmental and tax issues, ongoing efforts to explore various means for resolving asbestos
litigation, and other legal proceedings;
• our ability to protect our patents and other intellectual property, both domestically and internationally;
• interest rate and foreign currency exchange rate fluctuations, including the impact of possible currency devaluations in countries
experiencing high inflation rates;
• governmental laws and regulations affecting domestic and foreign operations, including, without limitation, tax
obligations and changes affecting the tax treatment by the U.S. of income earned outside the U.S. that may result
from pending and possible future proposals;
• any significant issues involving our largest wholesaler customers, which account for a substantial portion of our
revenues;
• the possible impact of the increased presence of counterfeit medicines in the pharmaceutical supply chain on our
revenues and on patient confidence in the integrity of our medicines;
• any significant issues that may arise related to the outsourcing of certain operational and staff functions to third
parties, including with regard to quality, timeliness and compliance with applicable legal requirements and industry
standards;
• changes in U.S. generally accepted accounting principles;
• uncertainties related to general economic, political, business, industry, regulatory and market conditions including,
without limitation, uncertainties related to the impact on us, our customers, suppliers and lenders and counterparties to
our foreign-exchange and interest-rate agreements of challenging global economic conditions and recent and possible
future changes in global financial markets; and the related risk that our allowance for doubtful accounts may not be
adequate;
• any changes in business, political and economic conditions due to actual or threatened terrorist activity in the U.S. and
other parts of the world, and related U.S. military action overseas;
• growth in costs and expenses;
• changes in our product, segment and geographic mix;
• our ability to successfully implement any strategic alternative that we decide to pursue with regard to our remaining
approximately 80% ownership stake in Zoetis Inc. and the impact thereof; and
• the impact of acquisitions, divestitures, restructurings, product recalls and withdrawals and other unusual items,
including our ability to realize the projected benefits of our cost-reduction and productivity initiatives, including those related
to our research and development organization.
We cannot guarantee that any forward-looking statement will be realized, although we believe we have been prudent in our plans and
assumptions. Achievement of anticipated results is subject to substantial risks, uncertainties and inaccurate assumptions. Should known or
unknown risks or uncertainties materialize or should underlying assumptions prove inaccurate, actual results could vary materially from past
results and those anticipated, estimated or projected. Investors should bear this in mind as they consider forward-looking statements.
We undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events or otherwise.
You are advised, however, to consult any further disclosures we make on related subjects in our Form 10-Q, 8-K and 10-K reports and our
other filings with the SEC.
Certain risks, uncertainties and assumptions are discussed here and under the heading entitled “Risk Factors” in Part I, Item 1A. of our Annual
Report on Form 10-K for the year ended December 31, 2012, which will be filed in February 2013. We note these factors for investors as
permitted by the Private Securities Litigation Reform Act of 1995. You should understand that it is not possible to predict or identify all such
factors. Consequently, you should not consider any such list to be a complete set of all potential risks or uncertainties.
This report includes discussion of certain clinical studies relating to various in-line products and/or product candidates. These studies typically
are part of a larger body of clinical data relating to such products or product candidates, and the discussion herein should be considered in the
context of the larger body of data. In addition, clinical trial data are subject to differing interpretations, and, even when we view data as
sufficient to support the safety and/or effectiveness of a product candidate or a new indication for an in-line product, regulatory authorities may
not share our views and may require additional data or may deny approval altogether.
Financial Risk Management
The overall objective of our financial risk management program is to seek to minimize the impact of foreign exchange rate movements and
interest rate movements on our earnings. We manage these financial exposures through operational means and by using various financial
instruments. These practices may change as economic conditions change.