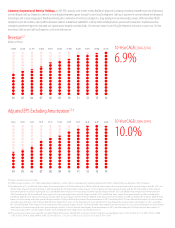
LabCorp 2014 Annual Report Download
Download and view the complete annual report
Please find the complete 2014 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
2014 ANNUAL REPORT
Table of contents
-
Page 1
2014 ANNUAL REPORT -
Page 2
... innovative solutions to healthcare stakeholders. LabCorp clients include physicians, patients and consumers, biopharmaceutical companies, government agencies, managed care organizations, hospitals and clinical labs. To learn more about Covance Drug Development, visit www.covance.com. To learn more... -
Page 3
...FORM 10-K [X] Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the fiscal year ended December 31, 2014 or [ ] Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the transition period from _____ to _____ Commission file... -
Page 4
...price on such date of the registrant's common stock on the New York Stock Exchange. Indicate the number of shares outstanding of each of the registrant's classes of common stock, as of the latest practicable date: 100.3 million shares as of February 20, 2015. DOCUMENTS INCORPORATED BY REFERENCE List... -
Page 5
...Changes on the Clinical Laboratory Business Company Strategy Laboratory Testing Operations and Services Testing Services Clients Payers Seasonality Investments in Joint Venture Partnerships Sales, Marketing and Client Service Information Systems Billing Quality Intellectual Property Rights Employees... -
Page 6
... of customers' reimbursement and health care economic consulting needs, and clinical trial support services. Covance's services are provided across multiple facilities in the United States, Europe and Asia. With the acquisition of Covance, which will operate as Covance Drug Development, the Company... -
Page 7
... method for non-US based employees to report, in local languages, a possible violation of LabCorp compliance policy or procedure or applicable law or regulation. Acquisition of Covance On February, 19. 2015, the Company completed its acquisition of Covance a leading drug development services company... -
Page 8
... with managed care companies; service capability and convenience offered by the laboratory; number and type of tests performed; connectivity solutions offered; and pricing of the laboratory's services. The Company believes that ongoing consolidation in the clinical laboratory testing business will... -
Page 9
... Health Insurance Exchanges and Accountable Care Organizations ("ACOs" or "ACO"). Despite the potential market changes discussed above, the Company believes that the volume of clinical laboratory testing will be positively influenced by several factors, including an expansion of Medicaid, managed... -
Page 10
... laboratory testing and most compelling value to its customers. Pillar One: Deploy capital to investments that enhance the Company's business and return capital to shareholders As discussed above, the Company completed its acquisition of Covance on February 19, 2015. In the fourth quarter of 2014... -
Page 11
..., as new developments in analytics and trending are changing existing ordering and workflow processes in the clinical laboratory industry. The Company's LabCorp Beacon® platform is a series of assets and functionalities that enhance the customer experience and provide an end-to-end lab solution... -
Page 12
... and highest value provider of laboratory services The Company maintains a constant focus on improving productivity and lowering costs throughout all phases of its operations from specimen collection to processing and testing, result reporting and billing. The Company's automation initiatives... -
Page 13
... complemented by Covance's robust end-to-end drug development services, enhance LabCorp's market leadership position in genomic testing. Beyond clinical trials, there are also many examples where companion diagnostics have moved into the commercial setting and are helping improve care, such as... -
Page 14
...a client order to indicate the tests to be performed and provide the necessary billing information. Some of the Company's PSCs also function as STAT labs, which are laboratories that have the ability to perform certain core tests and report results to the physician quickly. Each specimen and related... -
Page 15
Testing Services Core Testing The Company offers a broad range of clinical laboratory tests and procedures. Several hundred of these are frequently used in general patient care by physicians to establish or support a diagnosis, to monitor treatment or medication, or to search for an otherwise ... -
Page 16
... industry as well as approximately 140 genetic counselors and 6 medical geneticists to work with the Company's physician clients in optimizing patient outcomes. Endocrinology. The Company has emerged as a leading provider of advanced hormone/steroid testing including comprehensive services for the... -
Page 17
...managing chronic disease conditions. Development of New Tests Advances in medicine continue to fundamentally change diagnostic testing, and new tests are allowing clinical laboratories to provide unprecedented amounts of health-related information to physicians and patients. New molecular diagnostic... -
Page 18
... research centers and contract research organizations as part of required preclinical animal safety and efficacy testing. Through a variety of processes, technology and specifically constructed facilities, Covance Drug Development provides and specific pathogen free animals that meet its clients... -
Page 19
Covance Drug Development's capabilities provide clients the flexibility to conduct studies on a multinational and simultaneous basis. The data it provides is combinable and results in global clinical trial reference ranges because it uses consistent laboratory methods, identical reagents and ... -
Page 20
... hotlines, patient assistance programs and patient compliance REMS programs. Clients The Company provides testing services to a broad range of health care providers and other customers. During the year ended December 31, 2014, no client or group of clients under the same contract accounted for more... -
Page 21
...and mix of test work performed by the licensed providers in the province during the year. In 2014, the amount of the Company's cap revenue derived from the Ontario government sponsored health care plan was CN$202.1 million. Seasonality The Company experiences seasonality in its testing business. The... -
Page 22
... Meaningful Use requirements and ACO, Joint Commission and PQRS reporting requirements. Real time clinical alerts highlight gaps in care for patients and patient populations. These industry-leading, data driven services position LabCorp as a trusted partner to health care stakeholders, providing the... -
Page 23
... detailed in its quality improvement plan. Using quality assessment techniques, the Company's laboratories employ a variety of programs to monitor critical aspects of service to its clients and patients. In addition, the Company's supply chain management department provides oversight to monitor and... -
Page 24
... levels. As described below, these regulations concern licensure and operation of clinical laboratories, claim submission and reimbursement for laboratory services, health care fraud and abuse, security and confidentiality of health information, quality, and environmental and occupational safety... -
Page 25
...civil and criminal sanctions. Payment for Clinical Laboratory Services In 2014, the Company derived approximately 16.0% of its net sales directly from the Medicare and Medicaid programs. In addition, the Company's other clinical laboratory testing business that is not directly related to Medicare or... -
Page 26
... bill the program directly for covered tests performed on behalf of Medicare beneficiaries. State Medicaid programs are prohibited from paying more than the Medicare fee schedule limit for clinical laboratory services furnished to Medicaid recipients. Approximately 11.7% of the Company's revenue... -
Page 27
...April 1, 2015 and it not clear what policies Medicaid and Managed Care organizations may implement in response. The Company expects delays in the pricing and implementation of these new toxicology codes and it is unclear what impact will be experienced related to price and margins. Future changes in... -
Page 28
... to PHI. The HIPAA Privacy Rule amendment resulted in the preemption of a number of state laws that prohibit a laboratory from releasing a test report directly to the individual. The Company revised its policies and procedures to comply with these new access requirements and has updated its privacy... -
Page 29
... by some clinical laboratories and health care providers that raise issues under the federal fraud and abuse laws, including the Anti-Kickback Law. These practices include: (i) providing employees to furnish valuable services for physicians (other than collecting patient specimens for testing) that... -
Page 30
...; 2) payments by physicians to a laboratory for clinical laboratory services; 3) an exception for certain ancillary services (including laboratory services) provided within the referring physician's own office, if certain criteria are satisfied; 4) physician investment in a company whose stock is... -
Page 31
...clinical laboratory testing and may have a material adverse effect upon the Company's business. Government payers, such as Medicare and Medicaid, as well as insurers, including MCOs, have increased their efforts to control the cost, utilization and delivery of health care services. From time to time... -
Page 32
...vendors for disposal of such specimens. In addition, the federal Occupational Safety and Health Administration has established extensive requirements relating to workplace safety for health care employers, including clinical laboratories, whose workers may be exposed to blood-borne pathogens such as... -
Page 33
... ICD-10-CM is October 1, 2015. Clinical laboratories are typically required to submit health care claims with diagnosis codes to third party payers. The diagnosis codes must be obtained from the ordering physician. The failure of the Company, third party payers or physicians to transition within the... -
Page 34
...as point-of-care testing equipment that can be operated by physicians or other health care providers in their offices or by patients themselves without requiring the services of freestanding clinical laboratories. Development of such technology and its use by the Company's customers could reduce the... -
Page 35
... fix the cost of laboratory testing services for their enrollees. Under a capitated reimbursement mechanism, the clinical laboratory and the managed care organization agree to a per member, per month payment to pay for all authorized laboratory tests ordered during the month by the physician for the... -
Page 36
... payers to reduce the cost and utilization of clinical laboratory services, the Company needs to obtain and retain new customers and business partners. In addition, a reduction in tests ordered or specimens submitted by existing customers, without offsetting growth in its customer base, could impact... -
Page 37
..., perform testing, provide test results in a timely manner and/or bill the appropriate party. The Company is also continuing to enhance its LabCorp Beacon platform and could experience delays or deficiencies in the development process. Failure of the Company's information technology systems could... -
Page 38
... of the network or data gathering procedures could impede the processing of data, delivery of databases and services, client orders and day-to-day management of the business and could result in the corruption or loss of data. While certain operations have appropriate disaster recovery plans in place... -
Page 39
... from incorporating Covance Drug Development into its operations. The Company believes that the acquisition will provide an opportunity for revenue growth in development and commercialization of drugs and diagnostics, nutritional analysis and other areas, including a number of new business areas for... -
Page 40
... potential employment-related costs, including payments that may be made to certain Covance business' executives, filing fees, printing expenses and other related charges. There are also a large number of processes, policies, procedures, operations, technologies and systems that the Company intends... -
Page 41
... of service offerings; strengths in various geographic markets; price; technological expertise and efficient drug development processes; quality of facilities; ability to acquire, process, analyze and report data in an accurate manner; ability to manage large-scale clinical trials both domestically... -
Page 42
... that may undermine the usefulness of a trial or data from the trial or study or may delay the entry of a drug to the market. • Covance Drug Development also contracts with physicians, also referred to as investigators, to conduct the clinical trials to test new drugs on human volunteers. These... -
Page 43
... built or improved for the single purpose of providing clinical laboratory testing services. The Company believes that these facilities are suitable and adequate and have sufficient production capacity for its currently foreseeable level of operations. The Company believes that if it were unable to... -
Page 44
...payment data to DHCS in the summer of 2013. On March 28, 2014, Assembly Bill No. 1124 extended the implementation deadline of new regulations until June 30, 2016. Assembly Bill No. 1494 provides that until the new rates are set through this process, Medi-Cal payments for clinical laboratory services... -
Page 45
... this California legislation terminates the Company's reporting obligations (or obligation to provide a discount in lieu of reporting) under that agreement. In December 2014, DCHS announced at a stakeholder meeting the results of its analysis of payment data reported by laboratories in 2013 and its... -
Page 46
... by changing screening codes to diagnostic codes on laboratory test orders, thereby resulting in customers being responsible for co-payments and other debts. The lawsuit seeks injunctive relief, actual and punitive damages, as well as recovery of attorney's fees, and legal expenses. The Company will... -
Page 47
...Rita Varsam v. Laboratory Corporation of America DBA LabCorp, was filed in June 2014 in the Superior Court of California for the County of San Diego. In these lawsuits, the Plaintiffs allege on behalf of similarly situated employees that the Company failed to pay overtime, failed to provide meal and... -
Page 48
... and services provided by the Company to physicians who also received draw and processing/handling fees from competitor laboratories Health Diagnostic Laboratory, Inc. and Singulex, Inc. The Company is cooperating with the request. Under the Company's present insurance programs, coverage is obtained... -
Page 49
...on the New York Stock Exchange ("NYSE") under the symbol "LH." The following table sets forth for the calendar periods indicated the high and low sales prices for the Common Stock reported on the NYSE Composite Tape. High Year Ended December 31, 2013 First Quarter Second Quarter Third Quarter Fourth... -
Page 50
... each of the Company's common stock, the Standard & Poor's (the "S&P") Composite-500 Stock Index and the S&P 500 Health Care Index (the "Peer Group") and assuming that all dividends were reinvested. Comparison of Five Year Cumulative Total Return 12/2009 Laboratory Corporation of America $ Holdings... -
Page 51
... of December 31, 2014, the Company had outstanding authorization from the Board of Directors to purchase up to $789.5 million of Company common stock. The repurchase authorization has no expiration date. Following the announcement of the acquisition of Covance, Inc., the Company suspended its share... -
Page 52
... accounting firm. This data should be read in conjunction with the accompanying notes, the Company's consolidated financial statements and the related notes thereto, and "Management's Discussion and Analysis of Financial Condition and Results of Operations," all included elsewhere herein. Year Ended... -
Page 53
... the Company recorded a special charge of $6.2 related to the write-off of development costs incurred on systems abandoned during the year. The Company incurred approximately $25.7 in professional fees and expenses in connection with the acquisition of Genzyme Genetics and other acquisition activity... -
Page 54
... years of closing the transaction, 2015 results will be impacted by increased interest expense and lower margins from Covance. The Company manages its operations through two reportable segments: the Clinical diagnostics laboratory segment, which includes core testing as well as genomic and esoteric... -
Page 55
... and the benefit of fold-in acquisitions made in all years in both of the Company's segments, along with growth in the Company's managed care business and toxicology testing, partially offset by test and payer mix. During 2014, the impact of weather reduced the Company's revenues by an estimated $40... -
Page 56
... revenues resulting from the Medicare fee reductions, the impact of delays and denials of coverage of molecular pathology codes and sequestration in 2013, as mentioned above. Labor and testing supplies for the year ended December 31, 2014, comprise over 77.4% of the Company's cost of sales. Cost... -
Page 57
... (recorded in selling, general and administrative expenses) relating to fees incurred as part of its business process improvement initiative as well as one-time CFO transition costs. The Company also recorded $10.8 of costs related to the Covance acquisition, of which $4.8 is included in selling... -
Page 58
... cash-generating capability is one of the Company's fundamental strengths and provides substantial financial flexibility in meeting operating, investing and financing needs. On February 19, 2015, the Company completed its acquisition of Covance for approximately $6,200.0, pursuant to a definitive... -
Page 59
... insurance companies, managed care organizations, or Medicare and Medicaid will not approve reimbursement for these tests at a level commensurate with the costs of running the tests. Any or all of these circumstances could result in impairment in the value of the related capitalized licensing costs... -
Page 60
... letters of credit. The new revolving credit facility is permitted to be used for general corporate purposes, including working capital, capital expenditures, funding of share repurchases and certain other payments, and acquisitions and other investments. On January 30, 2015, the Company issued the... -
Page 61
... last business day of the calendar quarter, which is 5:00 p.m., New York City time, on Tuesday, March 31, 2015. If notices of conversion are received, the Company plans to settle the cash portion of the conversion obligation with cash on hand and/or borrowings under the new revolving credit facility... -
Page 62
... Revolving Credit Facility, the Company believes it has sufficient liquidity to meet both its anticipated short-term and long-term cash needs; however, the Company continually reassesses its liquidity position in light of market conditions and other relevant factors. Covance Drug Development Revenue... -
Page 63
...the number of patients to be enrolled or the type or amount of data to be collected. Contracts may range in duration from a few months to several years or longer depending on the nature of the work performed. In some installments as it reaches certain cases, Covance Drug Development bills the client... -
Page 64
...doubtful accounts Revenue is recognized for services rendered when the testing process is complete and test results are reported to the ordering physician. The Company's sales are generally billed to three types of payers - clients, patients and third parties such as managed care companies, Medicare... -
Page 65
... 31, 2014. Discount Rate The Company evaluates several approaches toward setting the discount rate assumption that is used to value the benefit obligations of its retirement plans. At year-end, priority was given to use of the Towers Watson Bond:Link model, which simulates the purchase of investment... -
Page 66
... estimated future economic conditions. These estimated liabilities are not discounted. The Company is self-insured (up to certain limits) for professional liability claims arising in the normal course of business, generally related to the testing and reporting of laboratory test results. The Company... -
Page 67
... compared to the book value of the reporting unit. The Company uses a market value approach for determining fair value and utilizes a number of factors such as publicly available information regarding the market capitalization of the Company as well as operating results, business plans, and present... -
Page 68
... or business models by managed care companies; failure to obtain and retain new customers or a reduction in tests ordered or specimens submitted by existing customers; difficulty in maintaining relationships with customers or retaining key employees as a result of uncertainty surrounding the Covance... -
Page 69
... benefits and synergies of the Covance acquisition could have a negative impact on the Company's cash position, levels of indebtedness and stock price; the inability of the Company and Covance to meet expectations regarding accounting and tax treatments related to the Covance acquisition; changes... -
Page 70
... the Company's principal executive officer and principal financial officer concluded that the Company's disclosure controls and procedures were effective as of the end of the period covered by this annual report. Changes in Internal Control Over Financial Reporting There was no change in the Company... -
Page 71
... to be filed with the Securities and Exchange Commission in connection with the Annual Meeting of Stockholders to be held in 2015 (the "2015 Proxy Statement") under the caption "Election of Directors." Information regarding executive officers is incorporated by reference to the Company's 2015 Proxy... -
Page 72
...is incorporated by reference to information in the 2015 Proxy Statement under the captions "Director Independence" and "Related Party Transactions." Item 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES The information required by this item is incorporated by reference to the 2015 Proxy Statement under... -
Page 73
PART IV Item 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES (a) List of documents filed as part of this Report: (1) Consolidated Financial Statements and Report of Independent Registered Public Accounting Firm included herein: See Index on page F-1 (2) Financial Statement Schedules: See Index on ... -
Page 74
... by reference to Exhibit 3.1 to the Company's current report on Form 8-K, filed with the Commission on March 31, 2008). Specimen of the Company's Common Stock Certificate (incorporated herein by reference to Exhibit 4.1 to the Company's Annual Report on Form 10-K for the fiscal year ended December... -
Page 75
... to Exhibit 10.4 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2004). National Health Laboratories 1988 Stock Option Plan, as amended (incorporated herein by reference to the Company's Registration Statement on Form S-1, filed with the Commission on July 9, 1990... -
Page 76
... Statement on Form S-8, filed with the Commission on January 21, 2003, File No. 333-102602). Laboratory Corporation of America Holdings Deferred Compensation Plan (incorporated herein by reference to Exhibit 10.22 the Company's Annual Report on Form 10-K for the fiscal year ended December 31... -
Page 77
... Lynch, Pierce, Fenner & Smith Incorporated, Wells Fargo Bank, National Association and Wells Fargo Securities, LLC (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on November 3, 2014). Amended and Restated Credit Agreement, dated as of December 19... -
Page 78
... the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized. LABORATORY CORPORATION OF AMERICA HOLDINGS Registrant By: /s/ DAVID P. KING David P. King Chairman of the Board, President and Chief Executive Officer Dated: February 26, 2015 76 -
Page 79
... Financial Officer) Chief Accounting Officer (Principal Accounting Officer) Director Director Director Director Director Director Director * F. Samuel Eberts III, by his signing his name hereto, does hereby sign this report on behalf of the directors of the Registrant after whose typed names... -
Page 80
... STATEMENTS AND SCHEDULE Page Report of Independent Registered Public Accounting Firm Consolidated Financial Statements: Consolidated Balance Sheets Consolidated Statements of Operations Consolidated Statements of Comprehensive Earnings Consolidated Statements of Changes in Shareholders' Equity... -
Page 81
... being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial... -
Page 82
PART I - FINANCIAL INFORMATION Item 1. Financial Information LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES CONSOLIDATED BALANCE SHEETS (In Millions) December 31, 2014 ASSETS Current assets: Cash and cash equivalents Accounts receivable, net of allowance for doubtful accounts of $211.6 ... -
Page 83
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF OPERATIONS (In Millions, Except Per Share Data) Years Ended December 31, 2014 2013 2012 6,011.6 $ 5,808.3 $ 5,671.4 3,808.5 3,585.1 3,421.7 2,203.1 2,223.2 2,249.7 1,198.2 1,128.8 1,114.6 76.7 81.7 86.3 17.8 21.8 ... -
Page 84
... Data) Years Ended December 31, 2014 2013 2012 $ 512.6 $ 575.4 $ 584.8 (89.5) (63.2) 31.3 (18.6) 42.1 7.3 (16.3) 16.4 - (124.4) (4.7) 38.6 (14.7) 47.7 1.5 (76.7) (3.2) 23.9 435.9 572.2 608.7 (1.4) (1.6) (1.7) $ 434.5 $ 570.6 $ 607.0 Net earnings Foreign currency translation adjustments Net benefit... -
Page 85
... of common stock under employee stock plans Surrender of restricted stock and performance share awards Conversion of zero-coupon convertible debt Stock compensation Income tax benefit from stock options exercised Purchase of common stock BALANCE AT DECEMBER 31, 2014 $ 11.7 - - 0.1 - - - - (0.5) 11... -
Page 86
... Net cash provided by operating activities CASH FLOWS FROM INVESTING ACTIVITIES: Capital expenditures Proceeds from sale of assets Proceeds from sale of investments Acquisition of licensing technology Investments in equity affiliates Acquisition of businesses, net of cash acquired Net cash used for... -
Page 87
... 2014 net revenues. Through a national network of laboratories, the Company offers a broad range of clinical laboratory testing services used by the medical profession in core testing, patient diagnosis, and in the monitoring and treatment of disease. In addition, the Company has developed specialty... -
Page 88
... test work performed by the licensed providers in the province during the year. The accounts receivable balances from the Ontario government sponsored health care plan were $22.4 and $33.2 at December 31, 2014 and 2013, respectively. The portion of the Company's accounts receivable due from patients... -
Page 89
...computation of diluted earnings per share because their impact would have been antidilutive: Years Ended December 31, 2014 2013 2012 - 0.1 2.4 Stock options Stock Compensation Plans The Company measures stock compensation cost for all equity awards at fair value on the date of grant and recognizes... -
Page 90
...related accounts with any resulting gain or loss reflected in the consolidated statements of operations. Capitalized Software Costs The Company capitalizes purchased software which is ready for service and capitalizes software development costs incurred on significant projects starting from the time... -
Page 91
... arising in the normal course of business, generally related to the testing and reporting of laboratory test results. The Company estimates a liability that represents the ultimate exposure for aggregate losses below those limits. The liability is discounted and is based on actuarial assumptions... -
Page 92
...the Company beginning January 1, 2017. The Company is currently evaluating the expected impact of the standard. In August 2014, the FASB issued a new accounting standard that explicitly requires management to assess an entity's ability to continue as a going concern, and to provide related financial... -
Page 93
... financial statements. 2. BUSINESS ACQUISITIONS On November 20, 2014, the Company completed its acquisition of LipoScience, Inc. ("LipoScience"), a provider of specialized cardiovascular diagnostic laboratory tests based on nuclear magnetic resonance (NMR) technology, for a purchase price of $5.25... -
Page 94
...enhance the Company's scientific differentiation and esoteric testing capabilities. On July 31, 2012, the Company completed its acquisition of MEDTOX Scientific, Inc. ("MEDTOX"), a provider of high quality specialized laboratory testing services and on-site/point-of-collection testing (POCT) devices... -
Page 95
... on all major business decisions as well as providing other participating rights to each partner. The equity method investments represent the Company's purchase of shares in clinical diagnostic companies. The investments are accounted for under the equity method of accounting as the Company does not... -
Page 96
... testing in future years at current levels. If the provincial government decides to limit or reduce its reimbursement of laboratory diagnostic services, it would have a negative impact on the profits and cash flows the Company derives from its Canadian joint venture. In December 2013, Alberta Health... -
Page 97
... information technology equipment was written off in 2014 with no impact to net income. 8. GOODWILL AND INTANGIBLE ASSETS The changes in the carrying amount of goodwill (net of accumulated amortization) for the years ended December 31, 2014 and 2013 are as follows: Clinical Diagnostics Laboratory... -
Page 98
... estimated to be $84.1 in fiscal 2015, $78.8 in fiscal 2016, $71.5 in fiscal 2017, $60.4 in fiscal 2018, $53.6 in fiscal 2019, and $462.2 thereafter. The Company paid $0.0, $0.0 and $2.5 in 2014, 2013 and 2012 for certain exclusive and non-exclusive licensing rights to diagnostic testing technology... -
Page 99
... Defined benefit plan obligation Restructuring reserves Self-insurance reserves Acquisition related reserves Deferred revenue Deferred compensation plan obligation Worker's compensation and auto Other 11. DEBT Short-term borrowings and current portion of long-term debt at December 31, 2014 and... -
Page 100
... letters of credit. The new revolving credit facility is permitted to be used for general corporate purposes, including working capital, capital expenditures, funding of share repurchases and certain other payments, and acquisitions and other investments. On January 30, 2015, the Company issued the... -
Page 101
...in certain circumstances, if one of the following conditions occurs: 1) If the sales price of the Company's common stock for at least 20 trading days in a period of 30 consecutive trading days ending on the last trading day of the preceding quarter reaches specified thresholds (beginning at 120% and... -
Page 102
... the last business day of the calendar quarter, which is 5:00 p.m., New York City time, on Tuesday, March 31, 2015. If notices of conversion are received, the Company plans to settle the cash portion of the conversion obligation with cash on hand and/or borrowings under the revolving credit facility... -
Page 103
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Dollars and shares in millions, except per share data) The scheduled payments of long term debt and future minimum lease payments for capital leases at the end of 2014 are summarized as follows: ... -
Page 104
...share data) During 2014, the Company purchased 2.7 shares of its common stock at a total cost of $269.0. As of December 31, 2014, the Company had outstanding authorization from the Board of Directors to purchase $789.5 of Company common stock. Following the announcement of the acquisition of Covance... -
Page 105
... for 2014 was unfavorably impacted by the the recording of a full valuation allowance for the write down of two of the Company's investments. The effective rate for 2013 was favorably impacted by the release of the capital loss valuation allowance and recording two years of the R&D tax credit. The... -
Page 106
... Company. The Company recognizes interest and penalties related to unrecognized income tax benefits in income tax expense. Accrued interest and penalties related to uncertain tax positions totaled $8.2 and $9.3 as of December 31, 2014 and 2013, respectively. During the years ended December 31, 2014... -
Page 107
..., 2014 there were 6.1 additional shares available for grant under the Plan. This Plan was approved by shareholders at the 2012 annual meeting. Stock Options The following table summarizes grants of non-qualified options made by the Company to officers, key employees, and nonemployee directors under... -
Page 108
... option. Groups of employees and non-employee directors that have similar exercise behavior with regard to option exercise timing and forfeiture rates are considered separately for valuation purposes. For 2014, 2013 and 2012, expense related to the Company's stock option plan totaled $6.9, $14.5 and... -
Page 109
...employees semi-annually in January and July of each year. Approximately 0.2 shares were purchased by eligible employees in 2014, 2013 and 2012, respectively. For 2014, 2013 and 2012, expense related to the Company's employee stock purchase plan was $4.0, $3.5 and $4.9, respectively. The Company uses... -
Page 110
...are an inevitable part of doing business in the health care field today. The Company believes that it is in compliance in all material respects with all statutes, regulations and other requirements applicable to its clinical laboratory operations. The clinical laboratory testing industry is, however... -
Page 111
... by changing screening codes to diagnostic codes on laboratory test orders, thereby resulting in customers being responsible for co-payments and other debts. The lawsuit seeks injunctive relief, actual and punitive damages, as well as recovery of attorney's fees, and legal expenses. The Company will... -
Page 112
...Rita Varsam v. Laboratory Corporation of America DBA LabCorp, was filed in June 2014 in the Superior Court of California for the County of San Diego. In these lawsuits, the Plaintiffs allege on behalf of similarly situated employees that the Company failed to pay overtime, failed to provide meal and... -
Page 113
... and services provided by the Company to physicians who also received draw and processing/handling fees from competitor laboratories Health Diagnostic Laboratory, Inc. and Singulex, Inc. The Company is cooperating with the request. Under the Company's present insurance programs, coverage is obtained... -
Page 114
... the Company Plan of $12.4, $8.4 and $11.3 in 2014, 2013 and 2012, respectively. The PEP covers the Company's senior management group. Prior to 2010, the PEP provided for the payment of the difference, if any, between the amount of any maximum limitation on annual benefit payments under the Employee... -
Page 115
... data) Year ended December 31, 2014 2013 2012 $ 3.4 $ 3.1 $ 2.4 16.4 14.7 14.9 (18.3) (17.3) (17.3) $ 6.6 8.1 $ 10.5 11.0 $ 12.1 12.1 Service cost for benefits earned Interest cost on benefit obligation Expected return on plan assets Net amortization and deferral Defined benefit plan costs Amounts... -
Page 116
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Dollars and shares in millions, except per share data) 2014 4.0% 7.0% 2013 4.8% 7.0% 2012 4.0% 7.0% Discount rate Expected long term rate of return The Company also updated the mortality ... -
Page 117
... (f) U.S inflation protection income (g) Total fair value of the Company Plan's assets Fair Value as of December 31, 2014 $ 4.1 64.1 25.3 7.9 36.0 10.3 115.1 6.3 269.1 Using Fair Value Hierarchy Level 1 Level 2 Level 3 $ 4.1 4.1 64.1 25.3 7.9 36.0 10.3 115.1 6.3 265.0 $ $ $ $ Fair Value... -
Page 118
...except per share data) The following assumed benefit payments under the Company Plan and PEP, which were used in the calculation of projected benefit obligations, are expected to be paid as follows: 2014 2015 2016 2017 2018 Years 2019-2023 Post-retirement Medical Plan The Company assumed obligations... -
Page 119
.... The following assumed benefit payments under the Company's post-retirement benefit plan, which reflect expected future service, as appropriate, and were used in the calculation of projected benefit obligations, are expected to be paid as follows: 2015 2016 2017 2018 2019 Years 2020-2024 Deferred... -
Page 120
... funds, which are indexed to externally managed funds. From time to time, to offset the cost of the growth in the participant's investment accounts, the Company purchases life insurance policies, with the Company named as beneficiary of the policies. Changes in the cash surrender value of the... -
Page 121
...blood, tissues and other specimens. Clinical diagnostics laboratory segment includes financial information related to the broad range of testing services that are reported primarily through the U.S. business operations. The other reportable segment includes the Company's non-U.S. clinical diagnostic... -
Page 122
...: Clinical laboratory diagnostics Other General corporate Total depreciation and amortization $ 180.3 9.8 44.2 234.3 $ 2013 171.2 9.2 42.1 222.5 $ 2012 169.1 8.1 40.3 217.5 $ $ $ 21. QUARTERLY DATA (UNAUDITED) The following is a summary of unaudited quarterly data: Year ended December 31, 2014... -
Page 123
...1, 2014. The unaudited pro forma results reflect certain adjustments related to past operating performance, acquisition costs and acquisition accounting adjustments, such as increased amortization expense based on the estimated fair value of assets acquired, the impact of the Company's new financing... -
Page 124
...II LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES VALUATION AND QUALIFYING ACCOUNTS AND RESERVES Years Ended December 31, 2014, 2013 and 2012 (Dollars in millions) Balance at beginning of year Year ended December 31, 2014: Applied against asset accounts: Allowance for doubtful accounts... -
Page 125
(THIS PAGE INTENTIONALLY LEFT BLANK) -
Page 126
(THIS PAGE INTENTIONALLY LEFT BLANK) -
Page 127
...Vice President, Investor Relations 336-436-5076 Center for Molecular Biology and Pathology 800-345-4363 Center for Occupational Testing 800-833-3984 Center for Esoteric Testing 800-222-7566 Paternity/Identity 800-582-0077 Covance Drug Development 609-452-8550 Websites www.labcorp.com www.covance.com... -
Page 128
Laboratory Corporation of America® Holdings 358 South Main Street Burlington, NC 27215 336-584-5171 www.labcorp.com