LabCorp 2014 Annual Report Download - page 94
Download and view the complete annual report
Please find page 94 of the 2014 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.F-15
During the year ended December 31, 2013, the Company acquired various other laboratories and related assets for approximately
$159.5 in cash (net of cash acquired). These acquisitions were made primarily to extend the Company's geographic reach in
important market areas and/or enhance the Company's scientific differentiation and esoteric testing capabilities.
On July 31, 2012, the Company completed its acquisition of MEDTOX Scientific, Inc. ("MEDTOX"), a provider of high
quality specialized laboratory testing services and on-site/point-of-collection testing (POCT) devices, for $236.4 in cash, excluding
transaction fees. The MEDTOX acquisition was made to extend the Company's specialty toxicology testing group and enhance
the Company's scientific differentiation and esoteric testing capabilities.
The MEDTOX purchase consideration has been allocated to the estimated fair market value of the net assets acquired, including
approximately $78.0 in identifiable intangible assets (primarily non-tax deductible customer relationships, trade names and
trademarks) with weighted-average useful lives of approximately 18 years ; $33.2 in deferred tax liabilities (relating to identifiable
intangible assets); and a residual amount of non-tax deductible goodwill of approximately $154.2.
During the year ended December 31, 2012, the Company also acquired various other laboratories and related assets for
approximately $95.8 in cash (net of cash acquired). These acquisitions were made primarily to extend the Company's geographic
reach in important market areas and/or enhance the Company's scientific differentiation and esoteric testing capabilities.
Contingent consideration liabilities associated with the Company's business acquisitions are recorded at fair value based upon
the estimated probability assessment of the earn-out criteria. Changes in the fair value of contingent consideration liabilities are
recognized in earnings until the arrangement is settled.
3. RESTRUCTURING AND OTHER SPECIAL CHARGES
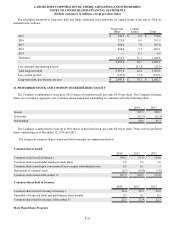
During 2014, the Company recorded net restructuring charges of $17.8. The charges were comprised of $10.5 in severance
and other personnel costs and $8.4 in facility-related costs primarily associated with general integration activities. These charges
were offset by the reversal of previously established reserves of $0.4 in unused severance and $0.7 in unused facility-related costs.
In addition, during 2014, the Company recorded $18.6 in consulting expenses (recorded in selling, general and administrative
expenses) relating to fees incurred as part of its business process improvement initiative ("Project LaunchPad") as well as one-
time CFO transition costs. The Company also recorded $10.8 of deal costs related to the announced acquisition of Covance, of
which $4.8 is included in selling, general and administrative expenses and $6.0 is included in interest expense.
During 2013, the Company recorded net restructuring charges of $21.8. The charges were comprised of $15.4 in severance
and other personnel costs and $9.5 in facility-related costs primarily associated with general integration activities. These charges
were offset by the reversal of previously established reserves of $0.7 in unused severance and $2.4 in unused facility related costs.
During 2012, the Company recorded net restructuring charges of $25.3. The charges were comprised of $16.2 in severance
and other personnel costs and $19.6 in facility-related costs primarily associated with the ongoing integration of Orchid and
Integrated Genetics Division (formerly Genzyme Genetics) and costs associated with the previously announced termination of an
executive vice president. These charges were offset by the reversal of previously established reserves of $6.3 in unused severance
and $4.2 in unused facility-related costs.
As part of the Clearstone integration, the Company also recorded a $6.9 loss on the disposal of one of its European subsidiaries
in Other, net under Other income (expenses) during 2012.
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)