LabCorp 2015 Annual Report Download - page 98
Download and view the complete annual report
Please find page 98 of the 2015 LabCorp annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Index
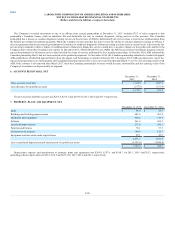
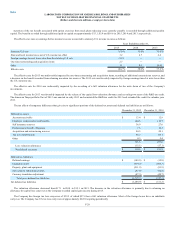
During the year ended December 31, 2013, the Company also acquired various other laboratories and related assets for approximately $159.5 in cash (net
of cash acquired). These acquisitions were made primarily to extend the Company's geographic reach in important market areas and/or enhance the
Company's scientific differentiation and esoteric testing capabilities.
Contingent consideration liabilities associated with the Company's business acquisitions are recorded at fair value based upon the estimated probability
assessment of the earn-out criteria. Changes in the fair value of contingent consideration liabilities are recognized in earnings until the arrangement is
settled.
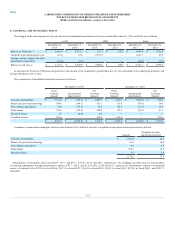
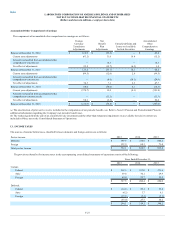
During 2015, the Company recorded net restructuring charges of $113.9; $39.2 within LCD and $74.7 within CDD. The charges were comprised of $59.2
in severance and other personnel costs and $55.8 in facility-related costs primarily associated with general integration activities. A substantial portion of
these costs relate to the planned closure of two CDD operations that serviced a minimum volume contract that expired on October 31, 2015. The charges were
offset by the reversal of previously established reserves of $1.1 in unused facility-related costs. Included within the facility-related charges noted above is a
$26.7 asset impairment charge relating to CDD lab and customer service applications that will no longer be used.
In addition, during 2015, the Company recorded $25.6 in consulting expenses (recorded in selling, general and administrative expenses) relating to fees
incurred as part of LCD's business process improvement initiative (Project LaunchPad) as well as one-time Covance integration costs and employee
compensation studies, along with $5.4 in short-term equity retention arrangements relating to the acquisition of Covance and $0.3 of accelerated equity
compensation relating to the previously disclosed retirement of a Company executive (all recorded in selling, general and administrative expenses). The
Company also incurred $5.7 relating to the wind-down of the minimum volume contract operations referred to in the previous paragraph. On February 9,
2016, the Company reached an agreement for the sale of the assets and business of one of these sites. As required by U.K. law, substantially all of the
employees were transferred with the business.
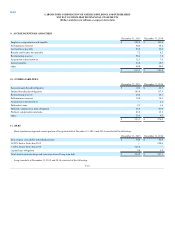
Additionally, the Company recorded $166.0 of deal costs related to the Acquisition, of which $113.4 is included in selling, general and administrative
expenses and $52.6 is included in interest expense. During 2015, the Company also recorded a non-cash loss of $2.3, upon the dissolution of one of its
equity investments, which is included in other, net expenses. During the fourth quarter, the Company paid $12.2 in settlement costs and litigation expenses
related to the resolution of a U.S. court putative class action lawsuit. In addition, the Company incurred $3.0 of non-capitalized costs associated with the
implementation of a major system as part of Project Launchpad.
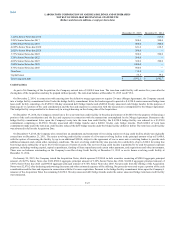
During 2014, the Company recorded net restructuring charges of $17.8. The charges were comprised of $10.5 in severance and other personnel costs and
$8.4 in facility-related costs primarily associated with general integration activities. These charges were offset by the reversal of previously established
reserves of $0.4 in unused severance and $0.7 in unused facility related costs.
During 2013, the Company recorded net restructuring charges of $21.8. The charges were comprised of $15.4 in severance and other personnel costs and
$9.5 in facility-related costs primarily associated with the ongoing integration of Orchid Cellmark, Inc. and the Integrated Genetics business (formerly
Genzyme Genetics) and costs associated with the previously announced termination of an executive vice president. These charges were offset by the reversal
of previously established reserves of $0.7 in unused severance and $2.4 in unused facility-related costs.
F-18