Humana 2004 Annual Report Download - page 47
Download and view the complete annual report
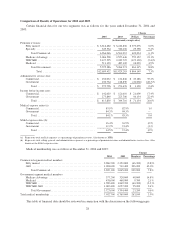
Please find page 47 of the 2004 Humana annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.medical utilization review, and customer service. Total capital expenditures, excluding acquisitions, were $114.1
million in 2004, $101.3 million in 2003, and $112.1 million in 2002. Excluding acquisitions, we expect our total
capital expenditures in 2005 to be approximately $115 million, most of which will be used for our technology
initiatives and improvement of administrative facilities. Proceeds from the sale of property and equipment relate
primarily to consolidating our service centers in Jacksonville and San Antonio including the sale of the
Jacksonville office tower in 2004 for $14.8 million and a San Antonio office building for $5.9 million in 2003.
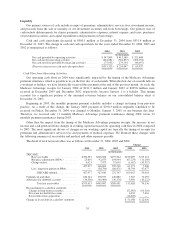
Cash Flow from Financing Activities
We repurchased 3.8 million common shares for $67.0 million at an average price of $17.83 per share in
2004, 3.7 million common shares for $44.1 million at an average price of $12.03 per share in 2003 and 6.4
million common shares for $74.0 million at an average price of $11.56 per share in 2002. Authorization for
additional common share repurchases expired in January 2005.
During 2003, we issued $300 million 6.3% senior notes due August 1, 2018 in order to term-out our short-
term debt and take advantage of historically low interest rates. In addition, during 2003 we received proceeds of
$31.6 million in exchange for new swap agreements. See Note 9 to the consolidated financial statements for more
detailed information regarding our borrowings and swap agreements.
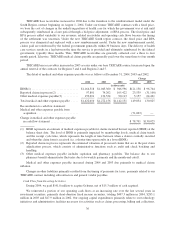
Credit Agreement
On September 29, 2004, we replaced our existing credit agreements with a new 5-year $600 million
unsecured revolving credit agreement which will expire in September 2009. We previously maintained two
unsecured revolving credit agreements consisting of a $265 million, 4-year revolving credit agreement and a
$265 million, 364-day revolving credit agreement.
Under the agreement, at our option, we can borrow on either a competitive advance basis or a revolving
credit basis. The revolving credit portion of the agreement bears interest at either a fixed rate or floating rate
based on LIBOR plus a spread. The spread, which varies depending on our credit ratings, ranges from 50 to
112.5 basis points. We also pay an annual facility fee regardless of utilization. This facility fee, currently 15 basis
points, may fluctuate between 12.5 and 37.5 basis points, depending upon our credit ratings. In addition, a
utilization fee of 12.5 basis points is payable for any day in which borrowings under the facility exceeds 50% of
the total $600 million commitment. The competitive advance portion of any borrowings will bear interest at
market rates prevailing at the time of borrowing on either a fixed rate or a floating rate basis, at our option.
The 5-year $600 million credit agreement contains customary restrictive and financial covenants as well as
customary events of default, including financial covenants regarding the maintenance of net worth, minimum
interest coverage, and maximum leverage ratios. At December 31, 2004, we were in compliance with all
applicable financial covenant requirements. The terms of this credit agreement also includes standard provisions
related to conditions of borrowing, including a customary material adverse effect clause which could limit our
ability to borrow. We have not experienced a material adverse effect, and we know of no circumstances or events
which would be reasonably likely to result in a material adverse effect. We do not believe the material adverse
effect clause poses a material funding risk to Humana in the future. We have other relationships, including
financial advisory and banking, with some of the parties to the new agreement.
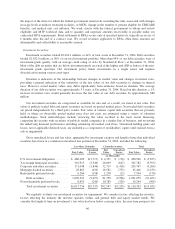
The maximum amount available for borrowing under the credit agreement was $594.6 million at December
31, 2004, reduced from the $600 million due to securing letters of credit of $5.4 million under the credit
agreement. No amounts have ever been drawn on these letters of credit. On February 16, 2005, we paid
approximately $450 million in cash for CarePlus including approximately $32 million of statutory capital and
surplus in excess of the minimum statutory requirement as well as estimated transaction costs. We financed the
transaction with $156 million of cash on hand and $294 million of borrowings under our credit agreement. After
the CarePlus transaction, we have $300.6 million of remaining borrowing capacity under the credit agreement.
37