Philips 2012 Annual Report Download - page 161
Download and view the complete annual report
Please find page 161 of the 2012 Philips annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
12 Group financial statements 12.11 - 12.11 26 27 28 29
Annual Report 2012 161
repayment of the loan. The trial in the case took place in December
2012 and after a period of post-trial briefing, a decision is expected in
the summer of 2013. One of the remaining issues in the case is whether
LPD’s alleged participation in the CRT cartel as determined by the
European Commission is a matter that should have been disclosed to
Mr. Vichi.
Optical Disc Drive (ODD)
On October 27, 2009, the Antitrust Division of the United States
Department of Justice confirmed that it had initiated an investigation
into possible anticompetitive practices in the Optical Disc Drive (ODD)
industry. Philips Lite-On Digital Solutions Corp. (PLDS), a joint venture
owned by the Company and Lite-On IT Corporation, as an ODD
market participant, is included in this investigation. PLDS is also subject
to similar investigations outside the US relating to the ODD market.
PLDS and Philips intend to cooperate with the authorities in these
investigations.
In July 2012, the European Commission issued a Statement of
Objections addressed to (former) ODD suppliers including the
Company. The European Commission granted the Company immunity
from fines, conditional upon the Company’s continued cooperation.
The Company responded to the Statement of Objections both in
writing and at an oral hearing.
Subsequent to the public announcement of these investigations in 2009,
the Company, PLDS and Philips & Lite-On Digital Solutions USA, Inc.,
were named as defendants in numerous class action antitrust
complaints filed in various federal district courts in the United States.
These actions allege anticompetitive conduct by manufacturers of
ODDs and seek treble damages on behalf of direct and indirect
purchasers of ODDs and products incorporating ODDs. These
complaints assert claims under federal antitrust law, as well as various
state antitrust and unfair competition laws and may involve joint and
several liability among the named defendants. These actions have been
consolidated by the Judicial Panel for Multidistrict Litigation for pre-
trial proceedings in the United States District Court for the Northern
District of California.
Consolidated amended complaints were filed on August 26, 2010 and
initially dismissed. Second Consolidated Amended Complaints were
filed on September 3, 2011. The defendants’ motions to dismiss the
Second Consolidated Complaints were denied on April 12, 2012 and
Philips has filed Answers to the Complaints of the direct and indirect
purchaser plaintiffs. Discovery is proceeding. Plaintiffs are expected to
file motions seeking to certify the putative classes of direct and indirect
purchasers under F.R.C.P. Rule 23 in April of 2013. Philips intends to
vigorously defend these actions.
The Company and certain Philips group companies have also been
named as defendants, in proposed class proceedings in Ontario,
Quebec, British Columbia, and Manitoba, Canada along with numerous
other participants in the industry. These complaints assert claims
against various ODD manufacturers under federal competition laws as
well as tort laws and may involve joint and several liability among the
named defendants. Philips intends to vigorously defend these lawsuits.
Due to the considerable uncertainty associated with these matters, on
the basis of current knowledge, the Company has concluded that
potential losses cannot be reliably estimated with respect to these
matters. These investigations and litigation could have a materially
adverse effect on the Company’s consolidated financial position, results
of operations and cash flows.
Philips Polska
In connection with an indictment issued by authorities in Poland in
December 2009 against numerous individuals, including three former
employees of Philips Polska sp. z.o.o., involved in the sale of medical
equipment to hospitals in Poland, Philips has been conducting a review
of certain activities related to sales of medical equipment for potential
violations of the U.S. Foreign Corrupt Practices Act (FCPA). Philips has
reported the review to US authorities, including the US Securities and
Exchange Commission, and is cooperating with US authorities in
connection with the review. Potential penalties for violations of the
FCPA and related statutes and regulations include monetary penalties
based, amongst others, on disgorgement of profits relating to the sale
of certain medical equipment in Poland. The discussions with the US
authorities are progressing. At this time the Company cannot indicate
when the matter will be resolved.
26 Cash from (used for) derivatives and securities
A total of EUR 47 million cash was paid with respect to foreign exchange
derivative contracts related to financing activities (2011: EUR 25 million
inflow; 2010: EUR 25 million outflow).
Cash flow from interest-related derivatives is part of cash flow from
operating activities. During 2012, there was no cash flow in relation to
these derivatives (2011: EUR nil million; 2010: EUR nil million).
27 Proceeds from non-current financial assets
In 2011, the sale of Philips’ interest in TCL Corporation (TCL) and
Digimarc generated cash totaling EUR 79 million.
In 2010, the redemption of TPV and CBAY convertible bonds generated
cash totaling EUR 239 million.
28 Assets in lieu of cash from sale of businesses
In 2012 Philips received certain financial instruments in exchange for
the transfer of its television business. At the date of this transaction the
fair value of these financial instruments involved an amount of EUR 17
million.
In 2011, the Company entered into four transactions with different
venture capital partners where certain incubator activities were
transferred in exchange for shares in separately established investment
entities. The investment entities represented a value of EUR 18 million
at the date that these transactions were closed.
In August 2010, the Company acquired a 49.9% interest in Shapeways
Inc. in exchange for the transfer of certain Consumer Lifestyle
incubator activities, which represented a value of EUR 3 million at the
date of the closing of that transaction.
29 Pensions and other postretirement benefits
Defined-benefit plans: pensions
Employee pension plans have been established in many countries in
accordance with the legal requirements, customs and the local situation
in the countries involved. The Company also sponsors a number of
defined-benefit pension plans. The benefits provided by these plans are
based on employees’ years of service and compensation levels. The
measurement date for all defined-benefit plans is December 31.
The Company’s contributions to the funding of defined-benefit pension
plans are determined based upon various factors, including minimum
contribution requirements, as established by local government, legal
and tax considerations as well as local customs.
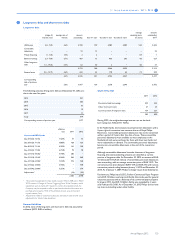
Summary of pre-tax costs for pensions and other
postretirement benefits
2010 2011 2012
Defined-benefit plans (105) 18 (38)
Defined-contribution plans including multi-
employer plans 114 120 142
Retiree medical plans 11 16 (14)
20 154 90
The 2012 cost were impacted by the recognition of a EUR 25 million
curtailment gain due to the accumulated reduction of employees as a
result of restructuring programs. A prior service cost gain of EUR 25
million was recognized in one of our major retiree medical plans. The
plan change reduced certain Company post retirement risks. In 2012 a
buy-out of the Swiss Pension Fund to an Insurance Company was
executed. The related decrease in DBO and assets for retirees is
included in the tables below as a settlement.
The 2011 costs were impacted by the recognition of EUR 18 million
curtailment gains mainly resulting from one of our defined-benefit plans
in which all remaining accrual of benefits was stopped and participants
were transferred to a defined-contribution plan. In the same plan a large
number of retirees opted for a higher yet non-indexed pension. The
resulting prior-service cost gain forms the larger part of the EUR 20
million prior-service cost gains recognized in 2011.